Summary
Background
The use of injectable progestin-only contraceptives has been associated with increased risk of HIV acquisition in observational studies, but the biological mechanisms of this risk remain poorly understood. We aimed to assess the effects of progestins on HIV acquisition risk and the immune environment in the female genital tract.
Methods
In this prospective cohort, we enrolled HIV-negative South African women aged 18–23 years who were not pregnant and were living in Umlazi, South Africa from the Females Rising through Education, Support, and Health (FRESH) study. We tested for HIV-1 twice per week to monitor incident infection. Every 3 months, we collected demographic and behavioural data in addition to blood and cervical samples. The study objective was to characterise host immune determinants of HIV acquisition risk, including those associated with injectable progestin-only contraceptive use. Hazard ratios (HRs) were estimated using Cox proportional hazards methods.
Findings
Between Nov 19, 2012, and May 31, 2015, we characterised 432 HIV-uninfected South African women from the FRESH study. In this cohort, 152 women used injectable progestin-only contraceptives, 43 used other forms of contraception, and 222 women used no method of long-term contraception. Women using injectable progestin-only contraceptives were at substantially higher risk of acquiring HIV (12·06 per 100 person-years, 95% CI 6·41–20·63) than women using no long-term contraception (3·71 per 100 person-years, 1·36–8·07; adjusted hazard ratio [aHR] 2·93, 95% CI 1·09–7·868, p=0·0326). HIV-negative injectable progestin-only contraceptive users had 3·92 times the frequency of cervical HIV target cells (CCR5+ CD4 T cells) compared with women using no long-term contraceptive (p=0·0241). Women using no long-term contraceptive in the luteal phase of the menstrual cycle also had a 3·25 times higher frequency of cervical target cells compared with those in the follicular phase (p=0·0488), suggesting that a naturally high progestin state had similar immunological effects to injectable progestin-only contraceptives.
Interpretation
Injectable progestin-only contraceptive use and high endogenous progesterone are both associated with increased frequency of activated HIV targets cells at the cervix, the site of initial HIV entry in most women, providing a possible biological mechanism underlying increased HIV acquisition in women with high progestin exposure.
Funding
The Bill and Melinda Gates Foundation and the National Institute of Allergy and Infectious Diseases.
Introduction
Hormonal contraceptives have empowered women to prevent unwanted pregnancies since being introduced in the 1960s. Unlike condoms, hormonal contraceptives do not need the cooperation of the male partner, can be taken discreetly, and are available in longacting formulations that are more than 99% effective. Injectable progestin-only contraceptives, including depot medroxy progesterone acetate (DMPA) and norethindrone enanthate (NET-EN), are the favoured form of con traception in sub-Saharan Africa, where they are used by more than 8 million women.1 Unfortunately, injectable progestin-only contraceptives do not provide protection against sexually transmitted infections and have been associated with a substantially increased risk of acquiring HIV in most but not all studies.2–5
In areas with both widespread injectable progestin-only contraceptive use and high HIV incidence, including many regions in sub-Saharan Africa, the questions of whether and how injectable progestin-only contraceptives affect HIV acquisition risk are of utmost importance. Decisions regarding the use of injectable progestin-only contraceptives must carefully balance the risk of HIV acquisition with that of unwanted pregnancy. The epidemiological studies driving these decisions are complicated by behavioural and demographic con founders that might mask the biological effect of injectable progestin-only contraceptives on HIV acquisition.6 Studies of the association between HIV acquisition and injectable progestin-only contraceptives use have not reached consistent conclusions, and although progestins have been shown to have a multitude of effects on immune function in vitro,7–10 whether these effects are manifest in vivo is unclear.11,12
Research in context.
Evidence before this study
We searched PubMed for articles published before July 7, 2015, using the search terms “depo provera” OR “dmpa” OR “nur isterate” OR “net-en” AND “HIV” and read those written in English. We examined relevant citations from the results of our search and identified studies that examined HIV acquisition and contraceptive use from an epidemiological perspective, with some, but not all, reporting a significant association. We also identified studies that assessed the effect of progestins on the function of immune cells in vitro, in animal models, in ex-vivo tissue, or in blood or assessed cohorts not at high risk of HIV acquisition. Some studies examined the biological associations with contraceptive use in high-risk cohorts but limited their observations to soluble immune mediators and did not find a concomitant increase in HIV acquisition risk.
Added value of this study
Previous studies examining the epidemiological risk of injectable progestin-only contraceptive use and HIV acquisition have variably been confounded by differences in behavioural risk factors reported between those using no long-term contraceptive and injectable progestin-only contraceptive users. These confounders might have masked potential biological effects of injectable progestin-only contraceptive use. Our study characterises a unique cohort of young women in which profiles of HIV risk factors had similar proportions across the cohort, so women using injectable progestin-only contraceptives and women using no hormonal contraception were similar in terms of HIV risk factors.
In this study, we identified an increased frequency of HIV target cells at the cervix of HIV-negative women using injectable progestin-only contraceptives and, in the same group of women, noted a significantly higher incidence of HIV acquisition in injectable progestin-only contraceptive users. We noted a similar pattern in HIV target cell frequency in the naturally high progestin state of the luteal phase in women not using long-term contraceptive, suggesting that progestins, both endogenous and exogenous, might be key mediators of target cell availability. Our work links epidemiological observations of HIV infection to the immunological effects of injectable progestin-only contraceptive use in the female genital tract and provides new mechanistic insight into the role of progestins in HIV acquisition risk in women.
Implications of all the available evidence
Our results, taken together with other studies, have cast further doubt about the safety of injectable progestin-only contraceptive in areas of high HIV incidence. Moreover, by concluding that a high progestin concentration, both in naturally cycling women and in injectable progestin-only contraceptive users, correlates with increased target cell availability at the cervix, we suggest that progestins might induce increased cervical HIV target cell frequency. This work provides new insight into the role of progestins in the female genital tract and might inform the development of biological prophylactics to reduce HIV acquisition for women.
We designed a prospective study and secondarily assessed the effects of progestins on HIV acquisition risk and the immune environment in the female genital tract, hypothesising that there is a biological mediator of the increased risk of HIV acquisition often observed in injectable progestin-only contraceptive users.
Methods
Study cohort and participants
HIV-negative women aged 18–23 years were recruited for the Females Rising through Education, Support, and Health (FRESH) study,13 an observational, prospective cohort study in Umlazi, South Africa (appendix). Participants were recruited by referral from three to four community organisations in Umlazi, South Africa, and community outreach events. We included women aged 18–23 years residing in Umlazi, South Africa, who were able to understand the information and consent forms, willing to adhere to study requirements, and willing to give voluntary consent for participation. We excluded individuals with unknown HIV status and unwilling to have HIV tests done; unwilling to consent to blood storage; enrolled in any other study that involves frequent blood sampling or might otherwise interfere with this study; with any conditions or conflict that is likely to prevent adherence to the study protocol and that might interfere with the outcome of the study, particularly adherence to the follow-up schedule, such as low likelihood to stay in or near Umlazi in the next 2–4 years or a chronic condition that requires regular blood sampling; pregnancy; and haemoglobin concentrations of less than 10 g/dL. The study protocol was approved by all local regulatory bodies (appendix). All participants provided written informed consent.
Procedures
Twice per week, participants attended classes focused on personal empowerment, job skills training, and HIV prevention, and had a finger prick blood draw for HIV RNA testing. Participants had a pelvic exam, phlebotomy, and completed a counsellor-administered HIV risk questionnaire every 3 months. The questionnaire asked participants whether they were using a family planning method, which method they were using, for how long they had been using that method, and whether they had a change in family planning method in the past 3 months. The questionnaire also addressed condom use and sexual behaviour. Study follow-up ranged from 3 months to 24 months. One nurse did all of the pelvic exams, during which endocervical cells were collected using a cytobrush (appendix). Samples were processed as described previously.13 Peripheral blood mononuclear cells were isolated from blood using a Ficoll gradient.
Consensus injectable progestin-only contraceptive use (appendix) was the primary covariate on which the primary analyses were based. Patient characteristics, high risk behaviour, and sexually transmitted infection status, assessed at the most recent follow-up, were also taken into account.
To detect sexually transmitted infections, HIV screening was done with HIV RNA PCR (Global Laboratories, Durban, South Africa), and positive results were repeated for confirmation. We tested for Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, HSV-1, and HSV-2 with a posterior fornix swab (appendix).
Plasma progesterone and oestradiol concentrations were measured by the Massachusetts General Hospital Clinical Laboratory Research Core using chemiluminescent microparticle immunoassays (Abbott Laboratories, Diagnostics Division, IL, USA). Progesterone values below the limit of detection (0·1 ng/mL) were read as half the minimum limit of detection (ie, 0·05 ng/mL). Oestradiol values below the limit of detection (10 pg/mL) were read as half the minimum limit of detection (ie, 5 pg/mL). Naturally cycling women were determined to be in the follicular or luteal phase of the menstrual cycle on the basis of their plasma progesterone concentration (follicular: progesterone ≤0·3 ng/mL or luteal: progesterone ≥1·2 ng/mL).
Cervical and peripheral blood cells were stained with titrated monoclonal antibodies (appendix) and analysed with FACS ARIA III or LSRFortessa (BD Biosciences, San Jose, CA, USA) and FlowJo software (TreeStar, Ashland, OR, USA), as described previously.13
An important determinant of HIV acquisition risk is the frequency of HIV target cells at the mucosal site of exposure in the female genital tract.14–16 To test the hypothesis that injectable progestin-only contraceptives increase target cell frequency at the superficial layers of the cervix, we did flow cytometric analysis on freshly collected matched cervical cells and peripheral blood. Because injectable progestin-only contraceptives affect endogenous hormone concentrations (appendix) and endogenous progesterone might affect immune function, we controlled for plasma progesterone con centrations; we analysed samples in which plasma progesterone concentration was at most 0·3 ng/mL. We assessed the concentration of 14 soluble cytokines in cervicovaginal lavage and the cervicovaginal microbiome, as described previously.13 Medroxy progesterone acetate concentration was measured in plasma as described previously17 and in the appendix.
Outcomes
The aims of our primary cohort study were identification and characterisation of host immune response and viral dynamics in acute HIV-1 infections. The secondary aims of our study included assessment of biological and behavioural correlates of HIV acquisition. Time to HIV-1 infection was defined as the time from enrolment to the first confirmed positive HIV RNA PCR result, or censored at the last day of contact. Assessment of HIV status was made at study entry and twice per week during follow-up with a rapid screening HIV RNA PCR assay followed by a confirmatory assay (ie, to confirm an initially positive test). Incidence rates are expressed as the number of HIV acquisition events per 100 person-years of follow-up.
Statistical analysis
This analysis was done using study records and samples from Nov 19, 2012, through to May 31, 2015. Descriptive measures were used to summarise the data. Continuous variables were summarised using median and interquartile range; categorical variables were summarised using frequency and percentage. Wilcoxon rank sum was used to compare continuous variables and Fisher’s exact test was used to compare categorical variables between groups. The HIV positivity (time to HIV-1 infection) data were analysed with the Kaplan-Meier method and the significance was tested by log-rank tests. Cox’s proportional hazards models were used to estimate hazard ratios (HRs) and their corresponding 95% CI. Two-sided p values are reported for all the statistical tests used in the analysis and p=0·05 was used as the cutoff for significance. R version 3.1.0 and Prism version 6 were used for the statistical analysis.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Nov 19, 2012, to May 31, 2015, we characterised 432 HIV-uninfected South African women from the FRESH study. In this cohort, 152 (35%) women used an injectable progestin-only contraceptive (DMPA or NET-EN), 43 women used another form of contraception, 15 women switched contraceptive methods during the study and were not included in the analyses comparing injectable progestin-only contraception users to women using no long-term contraception, and 222 (51%) women used no method of long-term contraception. Of the women using an injectable progestin-only contraceptive, 116 (76%) used DMPA and the remaining 36 (24%) used NET-EN. Use of injectable progestin-only contraceptives was self-reported but verified by measuring plasma progesterone and oestradiol con centrations in a randomly selected subset of 222 women and by directly measuring plasma medroxy progesterone acetate concentrations in a subset of 44 self-reported DMPA users (appendix). There was 97% and 93% concordance between reported DMPA use and measured use by progesterone and medroxy progesterone acetate quantification, respectively. Of the women who were using no long-term contraceptive, 129 were sampled during the follicular phase and 77 were sampled during the luteal phase, as determined by plasma progesterone concentration; the other 16 women were inconclusive based on progesterone measurements.
Despite the narrow age range and residency requirement dictated by study enrolment criteria, a few small demographic differences remained between women using injectable progestin-only contraceptives and women using no long-term contraceptive. Injectable progestin-only contraceptive users were 1 year older on average (p=0·022) and the age difference between the participant and her current sexual partner was 1 year greater for injectable progestin-only contraceptive users than for those using no long-term contraception (p=0·00036; table). No differences were seen in condom use, anal sex frequency, casual partner count, or bacterial vaginosis frequency. Injectable progestin-only contraceptive use was not associated with increased likelihood of having any sexually transmitted infections (table).
Table.
Baseline characteristics
| Overall participants* | Participants receiving DMPA or NET-EN* | Participants receiving no long-term contraception* | Other forms of contraception | p value of injectable progestin-only contraceptives vs no long-term contraception* | |
|---|---|---|---|---|---|
| Sexual demographics | |||||
|
| |||||
| Median age at enrolment | 21 (20–22) | 22 (20–23) | 21 (20–22) | 21 (21–22) | 0·02235 |
| Median age difference between participant and current sexual partner | 3 (1–5) | 4 (2–6) | 3 (1–4) | 4 (1–6) | 0·00036 |
|
| |||||
| Sexual behaviour† | |||||
|
| |||||
| Condom use in past 30 days | 0·1539 | ||||
| Always | 61/419 (15%) | 18/141 (12%) | 38/212 (18%) | 4/43 (9%) | ·· |
| Sometimes | 141/419 (34%) | 52/141 (35%) | 65/212 (31%) | 16/43 (37%) | ·· |
| Never | 102/419 (24%) | 41/141 (28%) | 44/212 (21%) | 16/43 (37%) | ·· |
| Not applicable (no sex) | 115/419 (27%) | 38/141 (25%) | 65/212 (30%) | 7/43 (16%) | ·· |
| Vaginal sex in past 7 days | 0·2504 | ||||
| 0 times | 240/421 (57%) | 80/151 (53%) | 132/214 (62%) | 20/41 (49%) | ·· |
| 1 time | 128/421 (30%) | 50/151 (33%) | 57/214 (26%) | 18/41 (44%) | ·· |
| 2 or more times | 53/421 (12%) | 21/151 (14%) | 25/214 (12%) | 3/41 (7%) | ·· |
| Vaginal sex episodes in past 30 days | 0·5212 | ||||
| 0 times | 115/420 (27%) | 38/151 (25%) | 65/213 (31%) | 7/41 (17%) | ·· |
| 1–5 times | 278/420 (66%) | 104/151 (69%) | 135/213 (63%) | 30/41 (73%) | ·· |
| More than 5 times | 27/420 (6%) | 9/151 (6%) | 13/213 (6%) | 4/41 (10%) | |
| Vaginal drying agent usage | 0·4194 | ||||
| Always | 5/385 (1%) | 2/142 (1%) | 1/190 (<1%) | 2/40 (5%) | ·· |
| Sometimes | 50/385 (13%) | 20/142 (14%) | 20/190 (11%) | 8/40 (20% ) | ·· |
| Never | 330/385 (86%) | 120/142 (85%) | 169/190 (89%) | 30/40 (75%) | ·· |
| Anal sex in past 30 days | 6/417 (1%) | 1/151 (<1%) | 5/210 (2%) | 0/41 | 0·2077 |
| Casual or new sex partner in past 30 days | 28/419 (7%) | 7/151 (5%) | 14/212 (7%) | 5/41 (12%) | 0·4286 |
|
| |||||
| Sexual health | |||||
|
| |||||
| Neisseria gonorrhoeae infection | 24/394 (6%) | 8/151 (5%) | 12/190 (6%) | 4/40 (10%) | 0·8179 |
| Chlamydia trachomatis infection | 53/394 (13%) | 13/151 (9%) | 29/190 (15%) | 7/40 (17%) | 0·0695 |
| Mycoplasma genitalium infection | 30/394 (8%) | 8/151 (5%) | 17/190 (9%) | 5/40 (13%) | 0·2170 |
| Trichomonas vaginalis infection | 34/394 (11%) | 12/151 (8%) | 12/190 (6%) | 9/40 (22%) | 0·6709 |
| HSV-1 infection | 4/394 (1%) | 2/151 (1%) | 2/190 (<1%) | 0/40 | 1·0000 |
| HSV-2 infection | 12/394 (3%) | 6/151 (4%) | 5/190 (3%) | 1/40 (3%) | 0·5468 |
| Bacterial vaginosis infection (Nugent score =7) | 102/394 (26%) | 33/151 (22%) | 54/190 (28%) | 12/40 (30%) | 0·1719 |
Data are median (IQR) or n/N (%), unless otherwise specified. p values are determined by Fisher’s exact test for categorical data and by Mann-Whitney for continuous data. These results represent the data collected at the most recent visit at which mucosal samples were collected, except when a woman became HIV positive before data was collected, in which case data is from the first HIV-positive visit (within a week of becoming infected). Age, age of sexual debut, and age of first partner were collected at the initial study visit. DMPA= Depot medroxyprogesterone acetate.
15 participants switched contraceptive during the study so were not included in columns where participants were analysed by contraceptive method.
Sexual behaviour information from one participant using NET-EN and ten participants using no long-term contraception was not available.
Despite intensive counselling and education, the HIV incidence in the cohort was 7·43 per 100 person-years (95% CI 4·59–11·36), with 24 women becoming infected by the time of analysis. In our study, the HIV incidence in injectable progestin-only contraceptive users (12·06 per 100 person-years, 6·41–20·63) was significantly higher than women using no long-term contraception (3·71 per 100 person-years, 1·36–8·07; HR 3·46, 95% CI 1·37–8·71, p=0·0084; figure 1, appendix). Demographic and behavioural differences between injectable progestin-only contraceptive users and non-users could not explain this increased risk; injectable progestin-only contraceptive use remained a significant predictor of HIV acquisition even after including the age difference between the participant and the partner as a covariate in a Cox proportional hazards model (adjusted hazard ratio [aHR] 2·93, 95% CI 1·09–7·86, p=0·0326).
Figure 1. Time to infection stratified by contraceptive usage.
Kaplan-Meier curve assessing HIV acquisition in women using injectable progestin-only contraceptives compared with those on no long-term contraceptive. p value determined by a log-rank test.
Having established the association between injectable progestin-only contraceptive use and HIV acquisition in this cohort, we hypothesised that biological correlates of injectable progestin-only contraceptive use could help to explain this epidemiological association. Hormonal contraceptive use has been shown to be associated with specific genital inflammatory cytokines18 and a lower prevalence of bacterial vaginosis,19 both of which might affect HIV acquisition risk.
After controlling for low progesterone and adjusting for multiple comparisons, no significant differences were identified in cytokine concentration in injectable progestin-only contraceptive users compared with those with no long-term contraception, by contrast with previous reports15 (appendix). In view of reports13 implicating the genital microbiome in inflammation and HIV acquisition risk, we also did bacterial 16S rRNA gene sequencing of nucleic acid isolated from cervical swabs. We identified no correlation between injectable progestin-only contraceptive use and bacterial populations in the female genital tract (appendix).
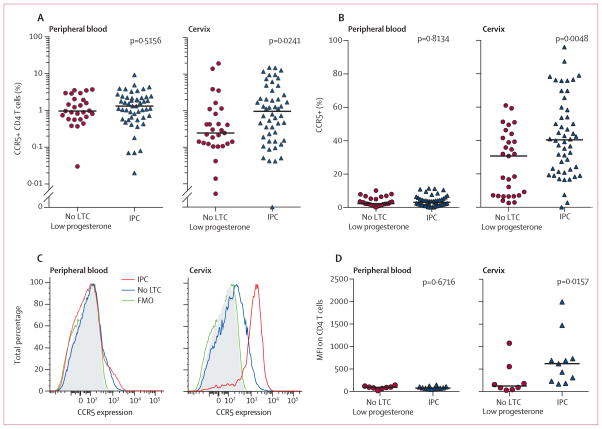
We identified significantly higher frequencies of HIV target cells (CCR5+ CD4 T cells) as a percentage of live CD45+ cells in the female genital tract of women using injectable progestin-only contraceptive compared with those using no long-term contraception (3·92 times higher, p=0·0241). We did not find this difference in peripheral blood cells in the same individuals (figure 2). We identified no difference in the number of live cervical CD45+ cells in the two groups. Cervical CD4 T cells of injectable progestin-only contraceptive users had higher CCR5 expression on the cell surface, as measured by the proportion of CD4 T cells expressing CCR5 (1·32 times higher, p=0·0048; figure 2), and higher median fluorescent intensity of CCR5 staining (p=0·0157; figure 2). Analysis of peripheral blood CD4 T cells in the same individuals did not show differences (figure 2). Oestradiol concentration did not correlate with CCR5+ CD4 T cell frequency or CCR5 expression (appendix).
Figure 2. HIV target cells in women using no LTC compared with women using an IPC.
CCR5+ CD4 T cells as a percentage of live CD45+ cells in the blood and cervix (A) and CCR5+ expression as a percentage of CD4 T cells in the blood and cervix (B). CCR5 expression levels on CD4 T cells as shown by a representative fluorescence-activated cell sorting histogram (C) and within all patients analysed (D). All participants had a plasma progesterone concentration of 0·3 ng/mL or less. p values were determined by the Mann-Whitney test. LTC=long-term contraception. IPC=injectable progestin-only contraception. FMO=Fluorescence minus one. MFI=Median fluorescence intensity.
Because activated target cells produce more viral particles per cell than resting target cells,20,21 which might accelerate viral dissemination,22 we next assessed target cell activation using the cell surface markers IL2Rα chain (CD25), HLA-DR, and CD38. Women using injectable progestin-only contraceptives had no difference in CD25+ target cells in the blood (figure 3) but had a significantly higher frequency of these cells in the female genital tract (p=0·0193; figure 3). Cervical target cells from injectable progestin-only contraceptive users did not co-express HLA-DR and CD38 at higher levels (appendix). Thus, injectable progestin-only contraceptive users had not only a higher frequency of cervical target cells but also more activated CD25+ target cells.
Figure 3. Frequency of activated HIV target cells in women using no LTC compared with women using an IPC.
HIV target cell activation as measured by CD25+CCR5+ CD4 T cells as a percentage of live CD45+ cells in the peripheral blood (A) and the cervix (B). All participants had plasma progesterone concentrations of 0·3 ng/mL or less. p values were determined by the Mann-Whitney test. LTC=long-term contraception. IPC=injectable progestin-only contraceptive.
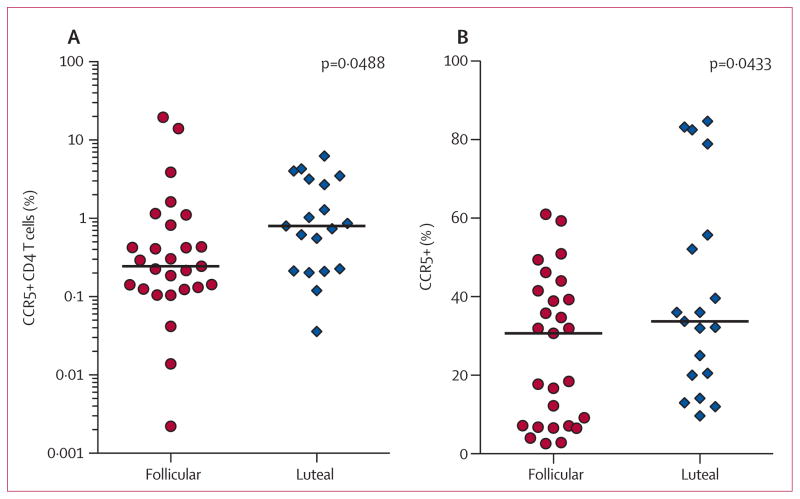
In view of the increased HIV target cells seen with exogenous progestin administration, we next examined whether natural states of high progestin, occurring during the luteal phase of the menstrual cycle, also exhibit female genital tract-specific changes in HIV target cells. Women in the luteal phase had a higher cervical target cell frequency (3·25 times higher, p=0·0488; figure 4) and more CCR5 expressing CD4 T cells (1·10 times higher, p=0·0433; figure 4) than women in the follicular phase.
Figure 4. Cervical HIV target cell frequency in the follicular and luteal phases of the menstrual cycle.
Frequency of cervical CCR5+ CD4 T cells of live CD45+ T cells (A) and CCR5 expression on cervical CD4 T cells (B) in women using no long-term contraceptive in the follicular versus luteal phases of the menstrual cycle. The follicular phase was defined as plasma progesterone concentration of 0·3 ng/mL or less; the luteal phase was defined as a plasma progesterone concentration of 1·2 ng/mL or above. p values were determined by the Mann-Whitney test.
Discussion
Defining the role of injectable progestin-only contraceptives in HIV acquisition is of utmost relevance, particularly in regions of the world where HIV transmission is highest. By prospectively following HIV-negative women in an area of high incidence, we first establish that injectable progestin-only contraceptives users in this cohort had significantly increased rates of HIV acquisition, an observation that has been shown previously in many2–4 but not all23,24 large cohorts. More importantly, we show that injectable progestin-only contraceptive use is associated with higher levels of HIV target cells in the cervix, providing a biological explanation for the reported increase in acquisition risk. We further show that a high level of endogenous progesterone is similarly associated with a higher frequency of cervical HIV target cells, supporting the conclusion that this cellular pattern is a biological effect of a high progestin state rather than an artifact of behavioural or demographic confounders associated with injectable progestin-only contraceptive use. These findings support a role for both endogenous and exogenous progestins in modulating the frequency of cervical target cells.
Behavioural and demographic confounders have previously obscured definitive analysis of an association between injectable progestin-only contraceptive use and HIV acquisition risk.2,6 Because participants were from a narrow age range and a single community, our cohort allowed pointed assessment of the link between injectable progestin-only contraceptive use and HIV acquisition. Of the potential confounders that we assessed, only age and age difference between a woman and her partner were significantly different between groups. These differences, although statistically significant, were very small in magnitude and of uncertain biological significance. In a Cox proportional hazard model, injectable progestin-only contraceptive use remained a significant predictor of HIV acquisition.
Previous epidemiological studies6 concluding that hormonal contraceptives increase risk have been faulted for a lack of coordinated investigation of plausible biological causes. We pursued the hypothesis that biological factors explain the increased HIV incidence in injectable progestin-only contraceptive users. In-vitro studies7–10 of human cells and tissue have suggested that endogenous reproductive hormones and exogenous progestins might directly affect immune cell function. Potential in-vivo effects have been less well described. We therefore assessed the immunological environment of the female genital tract in injectable progestin-only contraceptive users in our cohort, with particular focus on the frequency of HIV target cells at the site of exposure as a potential explanation for increased HIV acquisition.7,22 Women using injectable progestin-only contraceptives had a significantly higher frequency of activated cervical CCR5+ CD4 T cells. Activated target cells are not only more prone to infection but also support higher degrees of viral replication than resting cells.20,21 The increased frequency of activated cervical target cells therefore likely accelerates viral dissemination after exposure to HIV in the female genital tract.
Although the use of injectable progestin-only contraceptives has been of particular interest with respect to increased HIV acquisition risk, the luteal phase of the menstrual cycle, characterised by high endogenous progesterone, has also been described through model systems as a time of increased risk of HIV acquisition.25,26 We therefore examined cervical immune cells in women in different stages of the menstrual cycle. With the use of plasma progesterone concentrations to define follicular and luteal phases, we identified significantly higher HIV target cell frequency in the luteal phase. We conclude that women in any high progestin state—because of injectable progestin-only contraceptive use or naturally high endogenous progesterone—had increased HIV target cell frequency in the cervix compared with women in a low progestin state. No difference was reported in cervical target cell frequency between high progestin states (DMPA use, NET-EN use, and naturally high endogenous progesterone), suggesting a common immunomodulatory effect.
Our study had several limitations. The sample size was much smaller than previously published epidemiological studies examining the link between injectable progestin-only contraceptive use and HIV acquisition, which limited our ability to investigate the potential differential effects of DMPA and NET-EN use. Also, our analysis of immunological changes in the cervix during the follicular and luteal phase would have ideally been done by high frequency longitudinal sampling.
By showing the effect of injectable progestin-only contraceptives on HIV target cells in vivo, we provide a plausible biological mechanism for the significantly increased risk of HIV acquisition in women using injectable progestin-only contraceptives. We also noted similar immunological changes in the female genital tract of women in naturally high progestin states, which suggests that increased HIV acquisition risk is likely driven by both exogenous and endogenous progestin exposure. The significance of this analysis is two-fold. First, our findings in naturally cycling women further support that notion that differences in cellular phenotype and frequency are due to a biological effect, rather than to behavioural or demographic confounding. Second, if the target cell effect is truly mediated by both exogenous and endogenous progestins, then increased HIV acquisition risk might affect not only women using injectable progestin-only contraceptives but also naturally cycling women in the luteal phase. We also importantly identified that injectable progestin-only contraceptive use was not associated with increased genital inflammation or changes in bacterial communities, both of which have been associated with increased HIV acquisition in women, which suggests that the mechanism of increased HIV risk mediated by injectable progestin-only contraceptive is distinct from that of these other biological mediators, although these risk factors might converge on a common pathway of inducing increased HIV target cells in the female genital tract.
Our results suggest that a specific biological mechanism underlies the association between progestin exposure, particularly in the form of injectable progestin-only contraceptive use, and increased HIV acquisition risk in women. The effect of these findings on recommendations for women living in regions with high HIV incidence remains unclear. The potential risks of injectable progestin-only contraceptives will need to be weighed against those of other contraceptive alternatives and communicated clearly to women facing these choices. Together, our findings show new mechanistic insight into the role of progestins in HIV acquisition risk and suggest that efforts aimed at decreasing genital target cell availability in high progestin states might aid in the development of prophylactic strategies to improve prevention of HIV infection in women.
Acknowledgments
We thank the FRESH participants; Thandi Cele for performing the pelvic exams, Cynthia Matiwane, Hlengiwe Dladla, and Sjabulile Ngcobo for clinical support; and Bjorn Corleis, Christina Gosmann, and Antonella C Lisanti for critical feedback and assistance. This work was supported by the Collaboration for AIDS Vaccine Discovery of the Bill and Melinda Gates Foundation, the National Institute of Allergy and Infectious Diseases (1R01AI111918), and the International AIDS Vaccine Initiative (UKZNRSA1001). DSK received additional support from the Burroughs Wellcome Fund. MNA was supported by award number T32GM007753 from the National Institute of General Medical Sciences, and the Paul and Daisy Soros Fellowship. TN received additional support from the South African Research Chairs Initiative and an International Early Career Scientist Award from the Howard Hughes Medical Institute. The content is solely the responsibility of the authors and does not represent the official views of the funders.
Footnotes
Contributors
KEC, NP, MNA, and GSO did the flow cytometry. KEC, AMo, EHB, and GSO collected behavioural data, NI processed and managed clinical samples, MSG advised about statistical analyses. AMa and AL did mass spectrometry measurements of plasma DMPA. MNA, EHB, and BAB did the nucleic acid extraction and bacterial sequencing. MNA did the cervicovaginal lavage cytokine measurements. TN, KLD, BDW, and DSK designed and managed the clinical study. EHB, MNA, and DSK did the data analysis and prepared the manuscript. All authors discussed the results and commented on the report.
Declaration of interests
We declared no competing interests.
References
- 1.Ross JA, Agwanda AT. Increased use of injectable contraception in sub-Saharan Africa. Afr J Reprod Health. 2012;16:68–80. [PubMed] [Google Scholar]
- 2.Polis CB, Phillips SJ, Curtis KM, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90:360–90. doi: 10.1016/j.contraception.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women’s risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis. 2015;15:181–89. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med. 2015;12:e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noguchi LM, Richardson BA, Baeten JM, et al. Risk of HIV-1 acquisition among women who use different types of injectable progestin contraception in South Africa: a prospective cohort study. Lancet HIV. 2015;2:e279–87. doi: 10.1016/S2352-3018(15)00058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelton JD. Use of hormonal contraceptives and risk of HIV-1 transmission. Lancet Infect Dis. 2012;12:507–11. doi: 10.1016/S1473-3099(12)70112-3. [DOI] [PubMed] [Google Scholar]
- 7.Huijbregts RP, Michel KG, Hel Z. Effect of progestins on immunity: medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception. 2014;90:123–29. doi: 10.1016/j.contraception.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Africander D, Louw R, Verhoog N, Noeth D, Hapgood JP. Differential regulation of endogenous pro-inflammatory cytokine genes by medroxyprogesterone acetate and norethisterone acetate in cell lines of the female genital tract. Contraception. 2011;84:423–35. doi: 10.1016/j.contraception.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol. 2008;180:2029–33. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- 10.Huijbregts RP, Helton ES, Michel KG, et al. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013;154:1282–95. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell CM, McLemore L, Westerberg K, et al. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis. 2014;210:651–55. doi: 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sciaranghella G, Wang C, Hu H, et al. CCR5 expression levels in HIV-uninfected women receiving hormonal contraception. J Infect Dis. 2015;212:1397–401. doi: 10.1093/infdis/jiv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42:965–76. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnathan DG, Wetzel KS, Yu J, et al. Activated CD4+CCR5+ T cells in the rectum predict increased SIV acquisition in SIVGag/Tat-vaccinated rhesus macaques. Proc Natl Acad Sci USA. 2015;112:518–23. doi: 10.1073/pnas.1407466112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–23. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–57. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 17.Kleynhans L, Du Plessis N, Allie N, et al. The contraceptive depot medroxyprogesterone acetate impairs mycobacterial control and inhibits cytokine secretion in mice infected with Mycobacterium tuberculosis. Infect Immun. 2013;81:1234–44. doi: 10.1128/IAI.01189-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison C, Fichorova RN, Mauck C, et al. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr. 2014;66:109–17. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 19.Bradshaw CS, Walker J, Fairley CK, et al. Prevalent and incident bacterial vaginosis are associated with sexual and contraceptive behaviours in young Australian women. PLoS One. 2013;8:e57688. doi: 10.1371/journal.pone.0057688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meditz AL, Haas MK, Folkvord JM, et al. HLA-DR+ CD38+ CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo. J Virol. 2011;85:10189–200. doi: 10.1128/JVI.02529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–60. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–92. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 23.Crook AM, Ford D, Gafos M, et al. Injectable and oral contraceptives and risk of HIV acquisition in women: an analysis of data from the MDP301 trial. Hum Reprod. 2014;29:1810–17. doi: 10.1093/humrep/deu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy SI, Zheng W, Montgomery ET, et al. Oral and injectable contraception use and risk of HIV acquisition among women in sub-Saharan Africa. AIDS. 2013;27:1001–09. doi: 10.1097/QAD.0b013e32835da401. [DOI] [PubMed] [Google Scholar]
- 25.Saba E, Origoni M, Taccagni G, et al. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol. 2013;6:1081–90. doi: 10.1038/mi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vishwanathan SA, Guenthner PC, Lin CY, et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011;57:261–64. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]