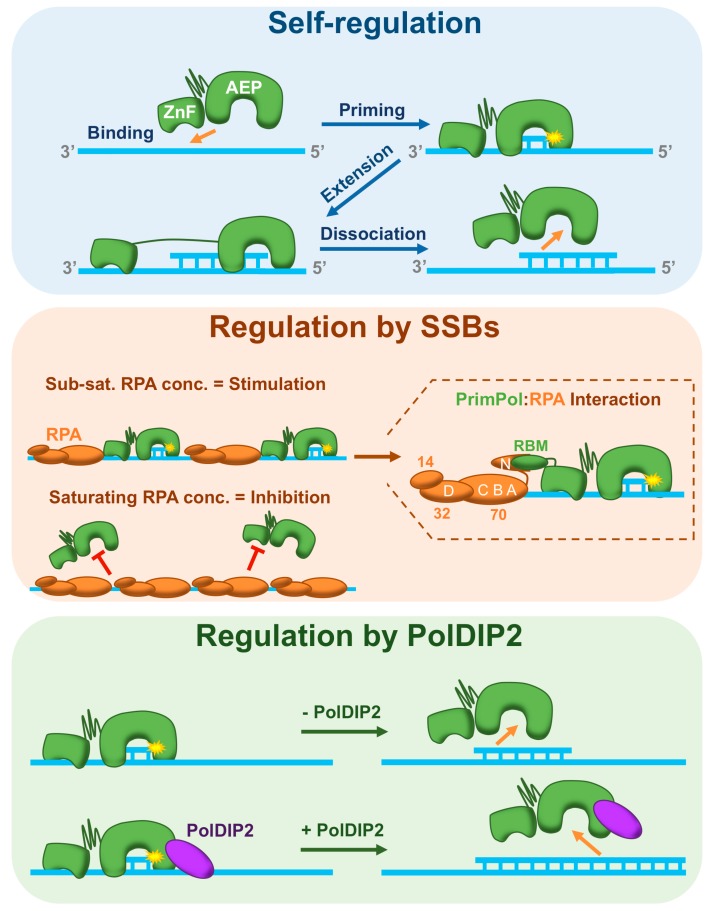
Figure 3.
Regulation of PrimPol by its ZnF domain and interacting partners. Top panel: PrimPol is inherently self-regulatory due to the restraining effect of its ZnF domain. The AEP and ZnF domains of PrimPol form a hinge-like structure, connected by a flexible linker. Binding of PrimPol to ssDNA is mediated by the ZnF domain, which binds 3’ relative to the AEP domain on the template strand. Binding of the ZnF stabilises the AEP domain, permitting primer synthesis. The AEP then extends the primer, but is restricted by the maximum distance it can move away from the ZnF. The enzyme subsequently dissociates leaving behind a short primer. This mechanism limits the processivity of the PrimPol; Middle panel: PrimPol is regulated by single-strand binding proteins (SSBs). At sub-saturating concentrations of RPA, the protein acts to recruit PrimPol to the ssDNA template, consequently stimulating primer synthesis. In vivo, this interaction is primarily mediated by PrimPol’s RBM-A, which binds to the basic cleft of RPA70N. At saturating RPA concentrations, when the ssDNA template is fully coated, PrimPol cannot gain access and primer synthesis is inhibited. This serves to limit where PrimPol can prime; Bottom panel: Polymerase-delta interacting protein 2 (PolDIP2 or PDIP38) enhances PrimPol’s primer extension activity by binding the AEP domain and stabilising it on DNA.