Abstract
Initiation of DNA Replication is tightly regulated in all cells since imbalances in chromosomal copy number are deleterious and often lethal. In bacteria such as Bacillus subtilis and Escherichia coli, at the point of cytokinesis, there must be two complete copies of the chromosome to partition into the daughter cells following division at mid-cell during vegetative growth. Under conditions of rapid growth, when the time taken to replicate the chromosome exceeds the doubling time of the cells, there will be multiple initiations per cell cycle and daughter cells will inherit chromosomes that are already undergoing replication. In contrast, cells entering the sporulation pathway in B. subtilis can do so only during a short interval in the cell cycle when there are two, and only two, chromosomes per cell, one destined for the spore and one for the mother cell. Here, we briefly describe the overall process of DNA replication in bacteria before reviewing initiation of DNA replication in detail. The review covers DnaA-directed assembly of the replisome at oriC and the multitude of mechanisms of regulation of initiation, with a focus on the similarities and differences between E. coli and B. subtilis.
Keywords: initiation of DNA replication, DnaA, oriC, regulation of DNA replication, Bacillus subtilis, sporulation
1. Introduction
The initiation of DNA replication is highly regulated and tightly coupled to the progression of the cell cycle to ensure that the frequency of initiation appropriately matches that of cell division. In this way, cells maintain correct chromosome copy number and ensure success in reproduction [1,2,3]. Under-replication leads to cells likely to be missing essential genetic information, whilst over-replication is highly disruptive of genetic regulatory processes and is frequently associated with disease and cell death.
Regulation of DNA replication is exerted primarily at the initiation step when an initiator protein binds to the origin of replication and promotes the assembly of a nucleoprotein complex from which replication forks diverge [4]. Much of our current understanding of DNA replication and its regulatory control in bacteria is derived from studies of the Gram-negative organism Escherichia coli, in which the initiator protein is DnaA and the origin is oriC. It is now clear that while the principles underlying the regulation of DNA replication initiation in E. coli apply to many other bacteria, the regulatory components are somewhat restricted in their distribution [2,5]. Thus the Gram-positive organism Bacillus subtilis has no known DNA replication regulators in common with E. coli, moreover, its bipartite origin of replication is strikingly different in arrangement to the continuous origin of E. coli [6]. Furthermore, when starved of nutrients, additional layers of DNA replication control are exerted in B. subtilis as it enters into the pathway of sporulation which is characterized by asymmetric cell division, and compartment-specific gene expression.
This review describes our current understanding of DNA replication initiation and its regulation in B. subtilis. As bacterial DNA replication is best understood in E. coli, we provide an overview of the replication phases of initiation, elongation and termination in this organism before highlighting differences that are known in Bacillus. This is followed by an in-depth coverage of initiation of DNA replication including the initiation machinery and the mechanisms of DnaA assembly at the origin, with particular emphasis on the roles of the Bacillus-specific components, DnaB and DnaD, in replisome assembly. Next, we discuss the activities of the regulators, YabA and Soj/Spo0J, during growth and Spo0A/Sda and SirA during sporulation. Finally their mechanisms of action are compared with those of the E. coli regulatory components. This review is concerned with the regulatory mechanisms of DNA replication initiation in B. subtilis and E. coli—it is not intended as a comprehensive review of the DNA replication mechanisms of all bacterial species.
2. DNA Replication
The process of DNA replication can be separated into three distinct phases: initiation, elongation and termination. During the initiation phase, a nucleoprotein complex assembles at the origin of replication. This induces localized DNA unwinding leading to helicase loading and recruitment of a full complement of replisome machinery. In the elongation phase, this replication machinery carries out template-directed DNA synthesis. This is continuous and processive on the leading strand, but discontinuous on the lagging strand where a more complex cycle of primer synthesis, strand elongation and fragment ligation takes place. Finally, during termination, DNA polymerization is halted at a specific termination site. Regulation of DNA replication occurs principally at the initiation stage, during or prior to the recruitment of the replication machinery.
2.1. Initiation of DNA Replication
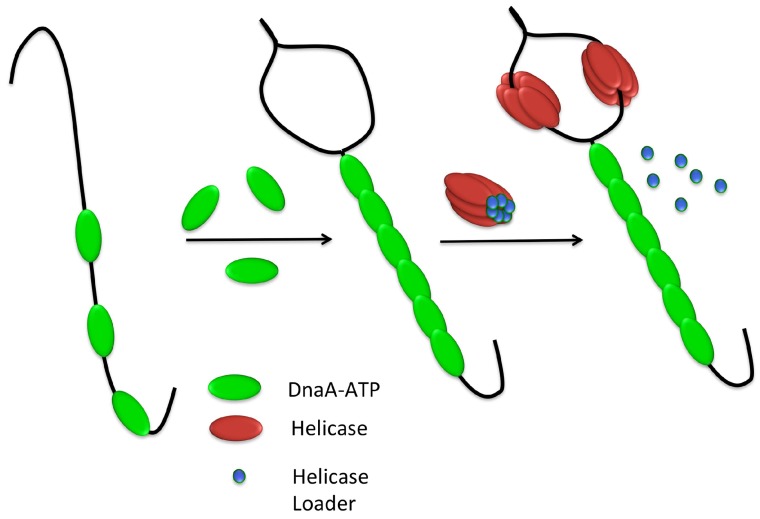
In bacteria, DNA replication is initiated by the binding of a protein initiator, DnaA, to the origin of replication, oriC (Figure 1). DnaA is understood to form a right-handed helical oligomer on the DNA [7,8] directed by its binding to a series of recognition sites within the origin termed DnaA-boxes [9]. The formation of this oligomer induces a localized unwinding of the DNA duplex within the origin at an AT-rich region termed the DUE (DNA Unwinding Element) [10,11]. DnaA then plays a role in recruiting the processive DNA helicase, named DnaB in E. coli or DnaC in B. subtilis [12], which is loaded onto the unwound single-stranded DNA (ssDNA) by a helicase loader, named DnaC and DnaI respectively [13] (Table 1).
Figure 1.
DNA replication initiation at oriC: DnaA (green) recognizes binding sites on oriC, forming a nucleoprotein complex which induces unwinding at the DNA unwinding element (DUE). The helicase loader then facilitates binding of the DNA helicase (red) as a prelude to recruitment and assembly of other components of the replication machinery. Figure inspired by [4].
Table 1.
The essential DNA replication initiation machinery of Bacillus subtilis and Escherichia. coli.
| Role in DNA Replication Initiation | B. subtilis | E. coli |
|---|---|---|
| Initiator | DnaA | DnaA |
| Helicase | DnaC | DnaB |
| DNA Remodelling | DnaB, DnaD | _ |
| Helicase Loader | DnaI | DnaC |
| Primase | DnaG | DnaG |
The helicase subsequently recruits the primase, DnaG, and the polymerase β-clamp, DnaN, which in turn recruits other components of the replication machinery in readiness for de novo DNA strand synthesis [14]. In B. subtilis, initiation requires two additional essential proteins, DnaD and DnaB [15], both of which possess DNA remodelling activities [16] and bind to the origin prior to helicase loading [17]. DnaD is thought to play a role in double-stranded DNA (dsDNA) melting, while DnaB appears to have a role in helicase loading. The essential components of the B. subtilis DNA replication machinery and their E. coli equivalents are listed in Table 1.
2.2. The Elongation Phase
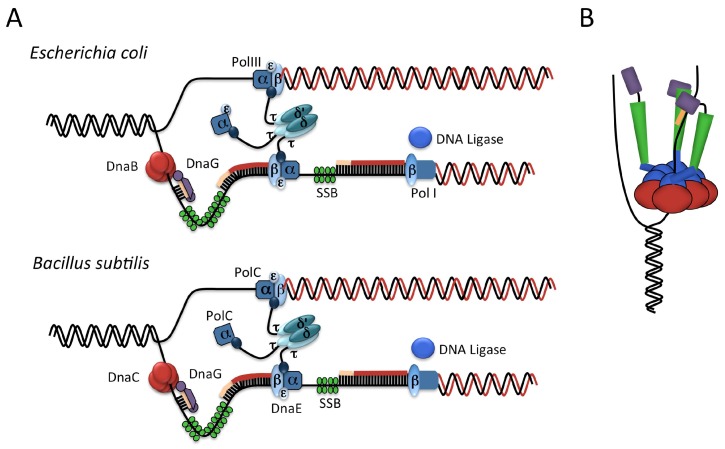
During the elongation phase of DNA replication, DNA is synthesized processively by the action of a large multi-subunit complex known as the replisome (Figure 2A). Based on single molecule biophysics studies in E. coli, the replisome consists of three DNA polymerase complexes, a hexameric DNA helicase, DNA primase (assumed from structural studies to comprise three subunits [18]), three processivity clamps, DnaN (two of which are associated with the core replisome), and a pentameric clamp loader complex [19].
Figure 2.
(A) Schematic representation of the E. coli and B. subtilis replisomes showing locations of the helicase, primase, DNA polymerase, the β-clamp and the clamp loader (τ3δδ’) at the replication fork. Figure adapted from [20] (B) Schematic of primase function. The helicase (red) unwinds the parental DNA, positioning a single strand ready for primer formation. The RNA polymerase binding domain (green) of one primase molecule forms a complex with the Zn binding domain (purple) of another primase molecule and single-stranded DNA (ssDNA) in order to synthesize the primer (orange). The C-terminal helicase-binding domain of the primase is shown in blue. Adapted from [24]
The helicase forms a homohexameric ring that is understood to sit at the head of the replication fork on the lagging strand of the template DNA. The helicase mechanically separates dsDNA by translocating along the lagging template strand in a process driven by ATP-hydrolysis. Separated DNA strands are coated in single-stranded DNA binding protein, SSB, which prevents the strands from re-annealing and offers protection to the ssDNA from nucleases [20,21,22].
The primase, DnaG, contains three functional domains; an N-terminal zinc-binding domain (ZBD), a central RNA polymerase domain (RPD) and a C-terminal helicase binding domain. Three DnaG molecules associate with the N-terminal domains of the helicase, positioned such that the primase captures the ssDNA which has been newly unwound by the helicase, ready for primer synthesis [23,24] (Figure 2B). The primase contains a groove that is thought to interact non-specifically with ssDNA, allowing the primase to track along the ssDNA and orientate it correctly for entry into the active site in the RPD, where primers are synthesized from available ribonucleoside tri-phosphate (rNTPs). The newly synthesized primer is extruded on the outside of the DnaB-DnaG complex, ready for handoff to SSB and DNA polymerase [23]. Whilst the RPD contains the catalytic site for RNA primer synthesis, the ZBD is responsible for modulating the activity of the RPD. Interestingly, the ZBD of DnaG regulates the RPD of an adjacent subunit in trans [25]. The RPD and ZBD from separate chains recognize the ssDNA template and initiate primer synthesis at specific trinucleotide recognition sites; with the ZBD increasing the catalytic activity of the trans RPD, as well as restricting processivity and primer length [25].
Strand extension in E. coli (Figure 2A) is carried out by DNA polymerase III (Pol III), which has an αεθ structure, where α is the catalytic subunit, ε is responsible for proofreading and θ is a non-essential subunit thought to stimulate the activity of ε. Pol III extends the primer with the assistance of the processivity clamp, DnaN (also known as the β-clamp). DnaN sits directly behind Pol III, as a closed ring on the DNA formed from two C-shaped subunits. DnaN binds across, rather than within, the major and minor grooves of duplex DNA, allowing the protein to slide along the DNA. In this way, the β-clamp enables the polymerase to synthesize up to 1000 bases a second [20,21,22]. The synthesis of each lagging strand Okazaki fragment requires the loading of a new β-clamp; thus the clamp loader complex forms part of the replisome machinery. The clamp loader is a pentameric complex with a subunit structure τ3δδ’. The τ subunit, the product of the gene dnaX, interacts with both the DNA helicase and Pol III—it is thought to play an architectural role at the replisome and couple DNA unwinding and DNA extension [20,21,22].
Elongation in B. subtilis occurs by a similar mechanism; however it uses two different, but related, replicative DNA polymerases, PolC and DnaE (Figure 2A). DnaE is more closely related to E. coli Pol III than PolC [26]. Both polymerases have been shown to be essential for lagging strand synthesis, whilst PolC is required for leading strand synthesis [27]. Each can extend DNA primers, but DnaE alone is able to extend the RNA primers produced by DNA primase. It is thus thought that DnaE extends the RNA primers with DNA before handing over to PolC for further strand synthesis [27]. This is analogous to systems in eukaryotes where DNA polymerase α extends RNA primers with DNA, before handing over to the lagging strand polymerase δ [20].
2.3. Termination of DNA Replication
The termination of DNA replication occurs at a termination locus positioned directly opposite oriC. In both B. subtilis and E. coli, replication termination is controlled by a polar mechanism in which the Ter site can be approached from a ‘permissive’ or ‘non-permissive’ direction. However, different mechanisms have evolved in each species.
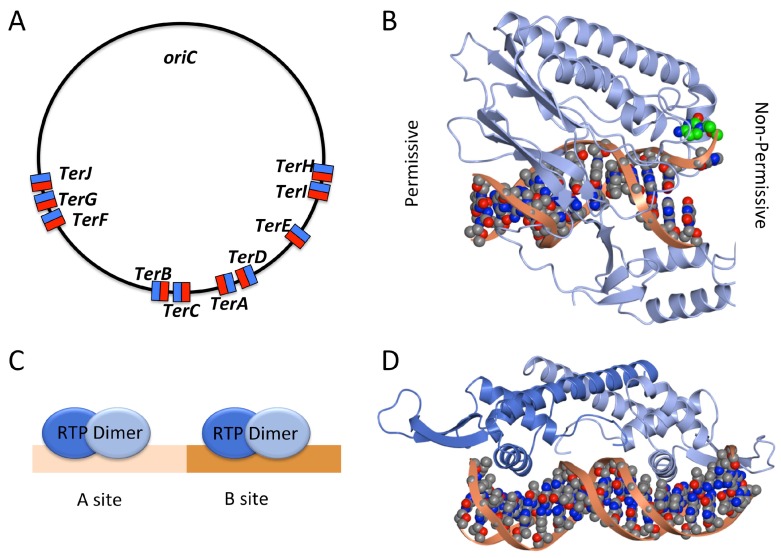
In E. coli, the locus directly opposite oriC is flanked on either side by five non-palindromic 23-bp sites, TerA-J (Figure 3), which bind the monomeric protein Tus (terminator utilisation substance) [28,29,30]. The orientation of these Ter sites dictates whether or not a travelling replication fork is able to pass the site or is halted in DNA replication [28,30]. Thus, a replication fork can bypass a Ter site unimpeded when travelling in the permissive direction, but is blocked when travelling in the non-permissive direction. For example, in Figure 3A, a replication fork travelling clockwise would bypass TerH, TerI, TerE, TerD and TerA, but would be halted at TerC (or failing that, at TerB, TerF, TerG or TerJ). Tus is a 36 kDa protein which specifically binds Ter sites in an asymmetric manner [31] (Figure 3B). Collision with the DNA helicase DnaB approaching from the permissive direction, causes Tus to rapidly dissociate. In contrast, when the approach is from the non-permissive direction, Tus-Ter forms a roadblock which prevents the translocation of DnaB and the associated replication fork [32]. Tus functions like a ‘molecular mousetrap’ at Ter. The trap is set by asymmetric binding of Tus to dsDNA in the non-permissive orientation, such that strand unwinding by the oncoming replication machinery ‘triggers’ the trap causing a specific cytosine base at position 6 of the Ter site to flip into a binding site on Tus. This gives rise to a ‘locked’ Tus-Ter complex (Figure 3B) which presents a roadblock to the progression of the replication fork [32,33].
Figure 3.
(A) Location and orientation of Ter sites in E. coli: permissive face shown in blue, non-permissive face shown in red; (B) Structure of the Tus-Ter complex (PDB code: 2EWJ) showing the permissive face (left) and the non-permissive face (right). On the non-permissive face a specific cytosine base (green) flips into Tus when double-stranded DNA (dsDNA) is unwound by the oncoming replication fork, creating a ‘locked’ complex; (C) Schematic image of two RTP dimers binding at the A and B sites of the Bacillus terminus region; (D) Structure of an RTP dimer bound to dsDNA (PDB code: 2EFW) with the sequence of a B-site region; one molecule displays a ‘wing up’ conformation (adjacent to the A site) and the other a ‘wing down’ conformation. (B), (D) and subsequent structural figures were rendered in CCP4MG [34].
In B. subtilis, the binding of two homodimers of the replication termination protein (RTP) at ‘A’ and ‘B’ sites within the Ter region is required to arrest replication (Figure 3C) [35,36]. The approach of the replication machinery from the ‘B’ site results in termination of replication (non-permissive direction) whilst approach from the ‘A’ site allows replication to continue (permissive direction). The crystal structure of a single RTP dimer bound to the native ‘B’ site has been shown to display asymmetry in the ‘wing’ region of the winged-helix domain [37] (Figure 3D). The protomer that lies proximal to the A-site shows a ‘wing-up’ conformation, while the other protomer displays a ‘wing down’ conformation, each making different contacts with the dsDNA. It is possible that this asymmetry gives rise to the ‘permissive’ and ‘non-permissive’ directions. However, A-site binding is also required to block replication fork progression, and A-site binding by RTP is co-operative following B-site binding [38]. The structural consequences of A-site binding are unknown and therefore the molecular basis of RTP action in replication termination remains unknown. Although the details of the E. coli and B. subtilis replication termination mechanisms vary, they appear to have evolved conceptually similar mechanisms for terminating replication in a direction specific manner.
3. Initiation of DNA Replication
3.1. Replication Origins
Replication origins have formed the topic of comprehensive recent reviews [39,40]. Knowledge of replication origins and how they encode DnaA-origin binding is key to the understanding of initiation mechanisms and how they are regulated. All origins harbor sequences that direct the formation of replication complexes, DNA unwinding, and species-specific regulatory activities. Conserved features of all bacterial replication origins include DnaA-box clusters and an AT-rich DUE. However across species, origins vary significantly in organization and length, including the number and spacing of DnaA-boxes and DnaA-box location with respect to the DNA unwinding elements. Of particular relevance to this discussion are two key differences between the origins of B. subtilis and E. coli: the genomic context of the origin and the number of intergenic regions that constitute oriC.
3.1.1. Genetic Context of Replication Origins
The location of the replication origin and its gene context are well conserved across bacterial species, with most flanked by, or containing, the dnaA gene [6,41]. The genes surrounding oriC and dnaA are also well conserved, consisting of the gene cluster rnpA-rpmH-dnaA-dnaN-recF-gyrB-gyrA with oriC residing in one or two intergenic regions adjacent to dnaA [6]. Unusually, the E. coli origin has undergone a major rearrangement resulting in a translocation of the origin 44 kb away from the dnaA gene and the rnpA-rpmH-dnaA-dnaN-recF-gyrB-gyrA cluster [41] so that it is instead flanked by the genes gidA and mioC [39]. Thus the origin of replication in B. subtilis may be more primitive than that of E. coli. Moreover, B. subtilis may provide a better model for bacterial replication origins in general [6].
3.1.2. Continuous and Bipartite Origins
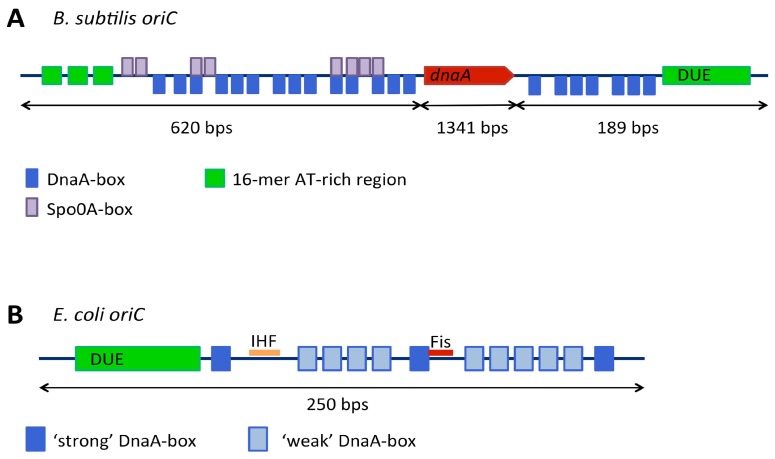
Origins are described as either continuous or bipartite according to whether all of the functional elements are contained in one or two intergenic regions respectively. For example, the origin of DNA replication in B. subtilis (Figure 4A) is bipartite, containing two DnaA-box clusters, separated by the dnaA gene [42,43]. In E. coli, the origin of replication is a continuous ≈250 bp element. (Figure 3B). The bipartite origin in B. subtilis has been shown to be important for proper replication initiation [36], although it is not clear how this difference in origin structure affects the assembly and architecture of the initiation machinery at the origin. During replication initiation, B. subtilis oriC forms looped structures which are thought to be a consequence of the bipartite nature of its origin [44]. These looped structures can also form using E. coli DnaA but E. coli DnaA is unable to unwind the B. subtilis origin. This supports the idea that a mechanism of DnaA binding at the origin leading to DnaA oligomerisation is applicable across bacterial species, as might be expected given the high conservation of DnaA. However, specific assembly and regulation of initiation encoded by each origin is likely to be species-specific.
Figure 4.
(A) B. subtilis origin of replication: DnaA-boxes are shown in blue, the dnaA gene in red, DNA unwinding element in green and Spo0A-boxes in purple; (B) The E. coli origin of replication: strong DnaA-boxes are shown in dark blue, weak DnaA-boxes in light blue, the DNA unwinding element in green and binding sites for accessory proteins integration host factor (IHF) and Fis in orange and red, respectively.
3.2. The DNA Replication Initiator, DnaA
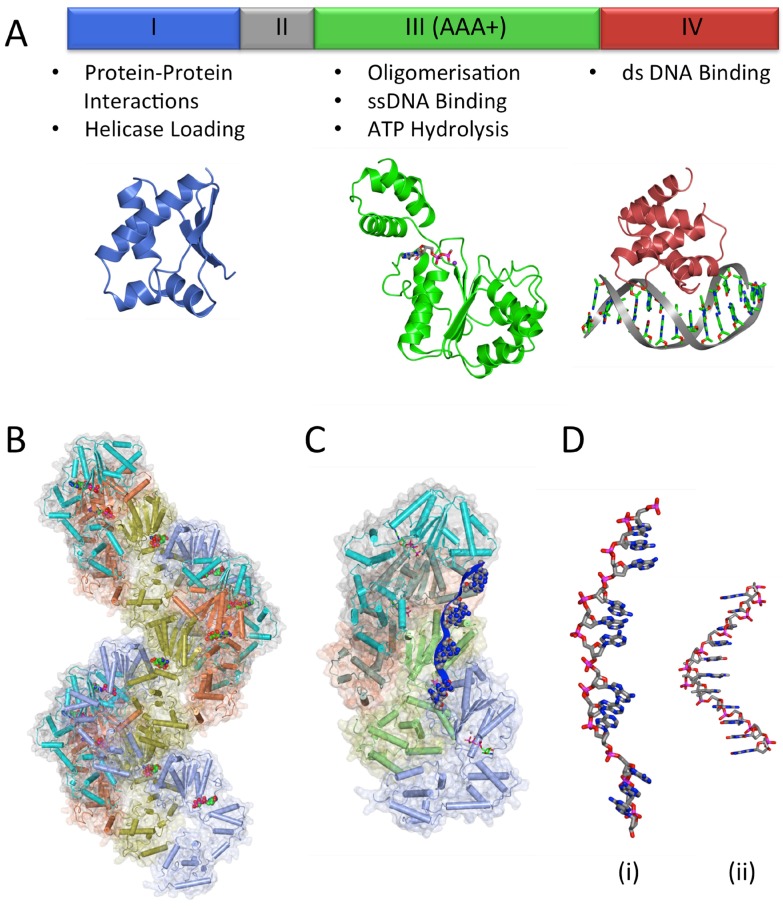
The initator DnaA is a member of the AAA+ ATPase family (ATPases associated with diverse cellular activities) and contains four distinct domains [45,46] (Figure 5A). In the cell, DnaA exists in both ATP- and ADP-bound forms [47]. DnaA–ATP is considered to be the ‘active’ form of the protein as this is required for oligomerisation at the origin [48,49], an event which triggers DNA unwinding and ultimately, assembly of the replisome. The C-terminal domain IV of DnaA is a dsDNA binding domain which is responsible for DnaA-box recognition [50,51]. The adjacent domain III contains Walker A and B motifs that are involved in ATP binding and hydrolysis. This domain plays a role in self-interaction/oligomer formation and in ssDNA binding [52]. Domain II of DnaA is poorly conserved and of variable length and considered to form a flexible linker which may play a role in controlling replication efficiency [53]. Finally, the N-terminal domain I is an ‘interaction domain’ which has been shown to interact with various protein regulators of DnaA across different organisms [54,55,56]. In E. coli, it also interacts with the helicase, DnaB [57], and has been suggested to play a role in the self-assembly of DnaA at the origin.
Figure 5.
(A) Schematic of DnaA showing domain architecture and structures. Domain I (PDB code: 4TPS) is shown in blue, domain II in gray, domain III (PDB code: 1L8Q) in green and domain IV (PDB code: 1JLV) in red. Figure adapted from [2]. (B) DnaA Domains III–IV bound to a non-hydrolysable ATP analogue (PDB code: 2HCB) form a spiral structure that is thought to mimic DnaA oligomerisation at the origin. A repeating pattern of DnaA protomers is shown in light blue, gold, coral and cyan; (C) ssDNA-binding mode of DnaA domains III–IV (PDB code: 3R8F). Separate DnaA protomers are shown in light blue, green, gray and cyan; (D) ssDNA binding by DnaA stretches the strand into an extended form (i) compared to B-form DNA (ii).
3.2.1. DnaA-Box Recognition by DnaA
The DnaA-boxes within the origin of replication vary in their affinity for DnaA, according to their similarity to a consensus binding sequence, and on the adenosine nucleotide bound state of DnaA [58,59]. In E. coli and B. subtilis, the consensus DNA-box is the nine-base-pair sequence, 5′-TTATNCACA-3′ [60].
An X-ray structure of DnaA domain IV bound to a consensus DnaA-box sequence revealed that DNA binding is mediated by a helix-turn-helix which interacts primarily with the major groove of the dsDNA, with additional contacts made in the adjacent minor groove [51] (Figure 5A). Base-specific interactions were observed at 8 of the 9 base pairs in the DnaA-box; the exception being the base pair at position 5, where there is no sequence preference [51]. Mutations at residues involved in base-specific interactions result in loss of DnaA-box binding specificity, or loss of DNA-binding altogether [61].
3.2.2. Variable Affinity of DnaA-Boxes
The DnaA-boxes at the origin can be either ‘strong’ or ‘weak’; where strong boxes bind both DnaA–ATP and DnaA–ADP with equal affinity and ‘weak’ boxes have a much greater relative affinity for DnaA–ATP [62]. In order for the helical DnaA oligomer to form at the origin and induce DNA unwinding, both strong and weak DnaA-boxes need to bind DnaA [63,64]. In the E. coli origin (Figure 4B), DnaA-boxes are distributed such that three strong boxes lie at either end of the origin and at its centre. As DnaA–ATP recruitment to the origin has been shown to be co-operative, these strong boxes are thought to form anchoring points from which the DnaA oligomer can grow [63,65]. In this model, DnaA–ATP is recruited to weak binding sites via co-operative interactions with DnaA–ATP molecules already bound to neighbouring sites [65].
3.2.3. DnaA Oligomerisation
Domains III–IV of Aquifex aeolicus DnaA have been shown to adopt an open spiral conformation [8] which likely mimics the right-handed helical oligomers ATP-bound DnaA forms at oriC [7] (Figure 5B). Adjacent protomers interact with one another via two clusters of conserved residues located on either side of the nucleotide binding pocket [8]. Significantly, DnaA–ADP cannot form this right-handed oligomer [7]; instead it appears to be monomeric [66]. The binding of ATP induces a small conformational change in the ATPase domain which allows an adjacent DnaA protomer to interact with the ATP via a conserved arginine residue known as an ‘arginine finger’. This interaction is significant in stabilising the DnaA helical filament [8] and similar ‘arginine finger’ interactions are frequently observed in other AAA+ ATPases [67]. Significantly, these observations provide a molecular explanation for why DnaA–ATP is the ‘active’ form of the initiatior.
In order to reconcile the Domain IV-DnaA-box binding mode with the DnaA helical oligomer formed by DnaA domains III–IV on dsDNA, a conformational change in the linker helix between domains III and IV has been invoked [51,68]. A significant kink in the linker helix is observed in the ATP-bound structure compared to the ADP-bound form (where the helix is straight) suggesting that the two domains are conformationally uncoupled and would be able to rotate with respect to one another to allow filament formation at the origin [8].
3.2.4. DNA Unwinding and ssDNA Binding
After the DnaA oligomer has formed at the origin, localized strand unwinding occurs at the DUE [69] (Figure 1). Based on structural work carried out with Aquifex aeolicus DnaA, unwinding is mediated by the DnaA-oligomer, which introduces positive writhe in the bound DNA [7]. Compensatory negative writhe at the DUE would facilitate DNA unwinding [4,8]. This unwound DNA is then stabilized by binding to the ssDNA binding site of DnaA located in the ATPase domain [69,70]. ATP-bound DnaA binds ssDNA in the same open spiral conformation displayed by DnaA domains III-IV [69]. In complexes of DnaA with single-stranded poly-(dA) DNA, each DnaA protomer binds three nucleotides, making multiple interactions with the DNA phosphodiester backbone. Each nucleotide triplet displays a normal B-form DNA conformation, but the triplets are separated by gaps of approximately 10 Å creating an overall extended form of DNA [69] (Figure 5D). This strand extension has been shown to be ATP-dependent in solution and is highly reminiscent of ssDNA binding displayed by the homologous recombination protein, RecA. The third base of each triplet is rotated however, making bases in the DnaA-bound strand discontiguous; this presumably prevents re-annealing of the strand at the origin [69] (Figure 5D).
Recently identified trinucleotide sequences within bacterial origins termed ‘DnaA-trios’ appear to be responsible for providing specificity of binding of DnaA to ssDNA, facilitating DNA-unwinding at the origin [71]. The trinucleotide motifs have the consensus sequence 3′-G/AAT-5′ and are separated from a proximal DnaA-box, or pair of boxes, by a GC-rich region. A DnaA molecule bound to the proximal DnaA-box via domain IV appears to be able to bind to the first of these DnaA-trio motifs via its AAA+ motif in domain III. Additional DnaA molecules interact with further DnaA-trio motifs, forming an oligomer on the ssDNA and facilitating DNA-unwinding [71].
3.2.5. Bacillus DnaA
B. subtilis DnaA has also been shown to form helical oligomers on both double and single stranded DNA [72], moreover, the DnaA–ATP form is required for co-operative binding to the origin [73]. Bacillus anthracis DnaA displays an ATP-dependent variable affinity for DnaA-box sequences [74]. Together these findings imply Bacillus DnaA functions at oriC in a similar manner to E. coli DnaA.3.2.6. The Role of DnaA Domains I–II in Initiation
DnaA domains I–II are not necessary for DnaA oligomerisation, or DnaA loading onto ssDNA [71]. Nevertheless DnaA domains I–II are required for initiation of replication [75]. DnaA domain I is known to interact with several regulators of DNA replication initiation; these include E. coli DiaA [76] and H. pylori HobA [55]—structural homologues and promoters of initiation in their respective organisms— and E. coli Hda [77] and B. subtilis SirA—two negative regulators of initiation [54]. In E. coli, domain I also interacts with the helicase DnaB [57,78,79] where it is thought to help correctly orientate the loading of DnaB at the origin. Domain I of DnaA has also been suggested to play a role in the self-assembly of DnaA at the origin [80,81]. It has a K homology (KH)-domain fold typically found in ssDNA binding proteins [82,83,84] (Figure 5A). In vitro DnaA domain I binds to single-stranded oriC DNA, albeit weakly, suggesting a potential role in binding ssDNA at the origin [84]. However, no ssDNA binding role has yet been demonstrated for DnaA domain I in vivo.
Domain II has been shown to be unstructured, consistent with a role as a flexible tether between domains I and III. It is not completely dispensable for DnaA function, but it is poorly conserved and varies significantly in length between organisms [46]. Two studies in E. coli have indicated that domain II contributes to the efficiency of initiation of replication. In one study, a spontaneous deletion in domain II allowed suppression of an over-initiation phenotype, suggesting that the deletion had reduced the efficiency of DNA replication initiation [85]. In another study, when deletions longer than 17–19 residues were made from domain II, the doubling time of cells harbouring this mutation was increased compared to wild type cells, suggesting the length of domain II contributed to the efficiency of DNA replication [53]. The same study defined the minimum length of domain II in E. coli to be 21–27 residues [53].
3.3. Helicase Loading
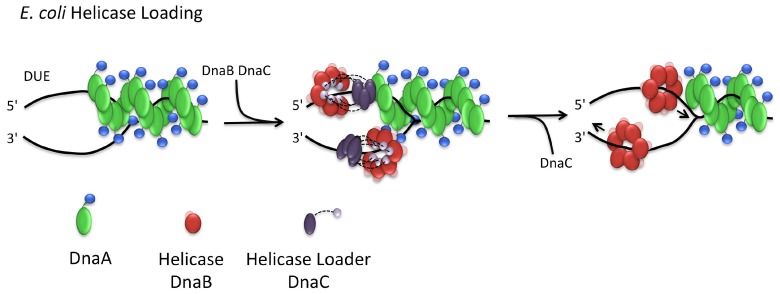
Following the unwinding of the DUE, a homohexameric DNA helicase is loaded onto single stranded DNA at the replication origin by the action of a helicase loader protein. In E. coli, the helicase, DnaB, is loaded onto the ssDNA by the helicase loader DnaC. This occurs via a ‘ring-breaking’ mechanism whereby DnaC forms a spiral oligomer which remodels the hexameric DnaB ring, producing a break in the ring large enough to allow loading onto ssDNA [86]. The recruitment of the DnaB–DnaC complex to the origin occurs by an interaction between the N-terminal domain of DnaA and the helicase, DnaB [12,84,87]. This interaction is thought to orient DnaB for loading onto the bottom strand of the DNA, while an interaction between the AAA+ domains of DnaA and DnaC is thought to recruit the complex in the right orientation for DnaB loading on the upper strand (Figure 6) [86,88].
Figure 6.
In E. coli, the initiator DnaA forms a helical oligomer during initiation which associates with the upper strand of the ssDNA. Following unwinding of the DUE, interactions between DnaA and DnaB or DnaC in the DnaC–DnaB complex are thought to correctly orientate DnaB for loading onto the bottom and top strands of DNA, respectively. Figure adapted from [88].
The primase, DnaG, is next recruited via an interaction with the N-terminal domain of DnaB. Subsequently, active primer formation appears to induce the dissociation of DnaC, in a step which is necessary for DnaB to begin to function as an active helicase. Release of DnaC appears to be dependent on the ATPase activity of DnaC which is thought to be induced by a conformational change in DnaB during primer formation [89]. DnaG interacts with the N-terminal domain of DnaB, while DnaC interacts with its C-terminal domain [90]. The loading of the helicase is important for the recruitment of the DNA polymerase clamp, DnaN. The clamp, in turn, recruits the DNA polymerase, in readiness for primer elongation [90,91].
Helicase loading in B. subtilis is thought to occur via a different mechanism known as ‘ring assembly’ [91]. In this model, the helicase loader, DnaI, facilitates the assembly of the helicase DnaC onto ssDNA [92,93]. In the presence of DnaI, pre-formed DnaC hexamers exhibit no helicase or translocase activity in contrast to monomeric DnaC which displays both helicase and translocase activities [92]. The helicase loader DnaI, like E. coli’s loader protein, contains an N-terminal helicase interaction domain and a C-terminal AAA+ domain [94]. The ATPase activity of the C-terminal domain of DnaI is stimulated in the presence of ssDNA, but only once inhibition by the N-terminal domain is overcome; binding of the N-terminal domain of DnaI to the helicase DnaC reveals a cryptic ssDNA binding site on the C-terminal domain [93]. It is thought that this then facilitates helicase loading onto ssDNA. Finally, the ATPase activity of the C-terminal domain may stimulate the release of DnaI once loading has occurred [93].
3.4. Bacillus Initiation Proteins DnaD and DnaB
Besides DnaA, DnaC (equivalent to E. coli DnaB), DnaI and DnaG, DNA replication initiation in B. subtilis requires the presence of two additional essential proteins, DnaD and DnaB [95,96]. A summary of their structure and function forms part of the discussion in an excellent recent review [6]. Both DnaD and DnaB are components of the replication initiation machinery at oriC [15] as well as components of the replication restart machinery which is DnaA-independent [97]. Both proteins exhibit DNA remodelling activities [16] and share structural similarity [96]. The B. subtilis initiation machinery assembles in a hierarchical manner, and DnaD and DnaB recruitment occurs between DnaA binding at oriC and the loading of the helicase, DnaC [17]. On binding to oriC, DnaD forms direct interactions with DnaA [98]. DnaD is required for the recruitment of DnaB and this, in turn, is then required for recruitment of DnaC-DnaI [17]. Together DnaB and DnaI are thought to function as a helicase loader [92].
The exact roles of DnaD and DnaB in replication initiation remain unclear. DnaD is able to untwist supercoiled DNA into an open looped form [99]. It forms tetramers which can assemble into large protein scaffolds that appear to mediate DNA loop formation and enhance melting of dsDNA [100]. The N-terminal domain of DnaD (DDBH1) is implicated in tetramer formation [101,102] with the C-terminal domain (DDBH2) involved in both double- and single-stranded DNA binding [100,102]. The full-length protein is required for DnaD to exhibit DNA looping and melting activities [100,102]. It is estimated that there are 3000–5000 DnaD molecules [103] in the cell and this relative abundance has led to the suggestion that DnaD plays a global role in DNA remodeling, beyond that required for DNA replication initiation [16]. In support of this idea, a study has shown that DNA remodeling by DnaD stimulates DNA repair by Nth endonucleases in response to DNA damage following treatment with H2O2 [104].
It is generally thought that DnaB acts together with DnaI to enable the loading of DnaC onto forked DNA [92]. However, studies [93,105] suggest that DnaI alone is sufficient to load the helicase onto DNA and that DnaB is required to recruit DnaC–DnaI to the origin [17] and that it acts to stimulate the helicase and translocase activities of DnaC in the presence of DnaI [92]. DnaB has also been implicated in the association of the DNA replication machinery with the cell membrane [95,106]. It has also been shown to laterally compact DNA—although it is not known how this contributes to its function [16].
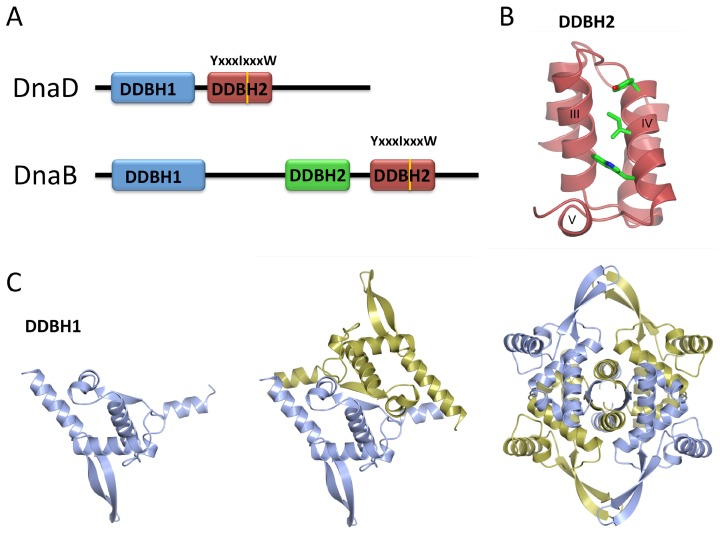
Although DnaD and DnaB show little sequence similarity, a Hidden Markov Model analysis identified two shared domains known as DDBH1 and DDBH2 (DDBH2 belongs to the PFAM domain: DnaB_2). DnaD has a DDBH1–DDBH2 architecture, whilst DnaB has a DDBH1–DDBH2–DDBH2 organization [96] (Figure 7A). The structure of the DDBH1 domain of DnaD revealed a winged helix domain with two additional structural elements: an N-terminal helix–strand–helix and a C-terminal helix [101] (Figure 7C). The β-strand of the helix–strand–helix was found to mediate interactions between DnaD molecules in both dimer and tetramer formation (Figure 7C). The C-terminal helix has been shown to be important in higher-order oligomerisation of these tetramers [101]. These structural elements appear to be present in DnaB DDBH1 [96] which has also been shown to form tetramers mediated by its N-terminus [16], suggesting that DnaB and DnaD share similar oligomerisation properties.
Figure 7.
(A) Diagram showing the architecture of DnaD and DnaB. Conserved DNA binding motif YxxxIxxxW is marked on the relevant DDBH2 domain; (B) Ribbon diagram of the DnaD DDBH2 domain from Streptococcus mutans (PDB code: 2ZC2). Tyrosine, Isoleucine and Tryptophan residues of the YxxxIxxxW motif are coloured by atom (carbon in green, nitrogen in blue and oxygen in red); (C) Ribbon diagram of DnaD DDBH1 domain from Bacillus subtilis (PDB code: 2V79) showing a winged helix with additional structural elements. Monomer, dimer and tetramer architectures are shown. Dimer and tetramer interactions are mediated by the β-strand of the additional helix–strand–helix. Figure inspired by [6].
DnaD’s DDBH2 domain has been shown to be involved in DNA-binding and in DNA-dependent higher-order oligomerization [102]. Two structures of the DDBH2 domain of DnaD homologues from Streptococcus mutans (PDB code: 2ZC2) and Enterococcus faecalis (PDB code: 2I5U) show a compact helical structure with four longer helices I–IV and a shorter fifth helix (V) of only 4 residues (Figure 7B). Although residues following helix V are poorly conserved across DnaD homologs, secondary structure prediction and analysis of the B. subtilis DnaD DDBH2 domain by NMR suggests that helix V is extended by a further seven residues [96]. Helix V is followed by a region at the C-terminus that is predicted to be disordered. A YxxxIxxxW motif residing in helix IV, the poorly conserved helix V and the C-terminal unstructured region [96] have been shown to be important for ssDNA binding. These structural elements appear to be conserved in the second of the DDBH2 domains of DnaB [96]. This domain has been implicated in dsDNA and ssDNA binding as well as in higher-order oligomerization [107]. Again, this suggests that the domains play similar roles in the respective proteins.
A DnaB (1–300) fragment encompassing DDBH1–DDBH2 (missing the C-terminal DDBH2 domain) forms tetramers and binds ssDNA [96,107]. Interestingly, C-terminally truncated cytosolic forms of DnaB have been observed during the mid–late growth phase. Full length DnaB alone is observed at oriC, thus proteolysis may be regulating DnaB function [107]. It is unclear whether the truncated version of the protein has a discrete function [107], however the different DNA binding capabilities of the DDBH2 domains of DnaB may be important in differentiating the functions of the full-length and truncated versions of DnaB.
3.5. Regulation of DNA Replication
3.5.1. During Vegetative Growth in B. subtilis
YabA
YabA is a negative regulator of DNA replication in Bacillus subtilis, affecting both the timing and synchrony of DNA replication in vegetatively growing cells [108]. Deletion of yabA causes an increased frequency of initiation events and asynchronous DNA replication [108] as well as a growth phenotype associated with increased initiation events [109,110]. YabA interacts with both the replication initiator, DnaA, and the DNA polymerase clamp, DnaN [109,110]. Mutations of YabA affecting the interaction with either DnaA or DnaN have been shown to exhibit an over-initiation phenotype similar to that in ΔyabA cells. This suggests that both interactions are important for replication regulation [110].
Expression of yabA genes encoding DnaA-loss-of-interaction or DnaN-loss-of-interaction mutations disrupts the formation of YabA foci at mid-cell, where it is assumed that YabA is co-localized with the replisome. Significantly, however, co-expression of DnaA-loss-of-interaction and DnaN-loss-of-interaction YabA-mutants restores YabA foci, presumably through a hetero-oligomer produced by the two mutants. This implies that both interactions are simultaneously required for YabA localization at the replisome [110,111].
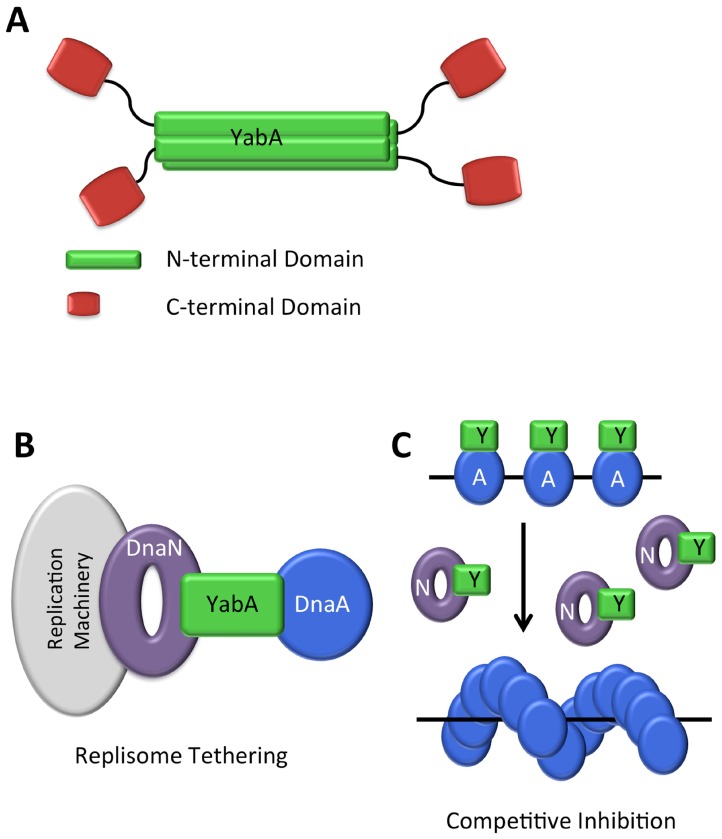
YabA forms tetramers through interactions of N-terminal coiled-coil domains to form an intermolecular 4-helix bundle. This provides a structural scaffold from which four C-terminal Zn-binding domains project. These are connected to the N-terminal domain by a flexible linker and they appear to be independent domains [111] (Figure 8A). The determinants on YabA for DnaA and DnaN interactions lie within these C-terminal domains. Significantly, yeast three-hybrid experiments show that full-length YabA is able to interact simultaneously with DnaA and DnaN [110], whereas the C-terminal domain alone cannot [111]. Thus, the YabA tetramer organization facilitates simultaneous interactions with DnaA and DnaN.
Figure 8.
(A) Schematic of YabA tetramer structure: YabA N-terminal domains (green) form a 4-stranded coiled coil structure. Pseudo-monomeric C-terminal Zn-binding domains (red) are attached by flexible linkers; (B) Replisome tethering model. YabA tethers DnaA to the replisome via an interaction with both DnaA and DnaN, titrating DnaA away from the replication origin; (C) Co-operative inhibition model. YabA (Y) inhibits the cooperative binding of DnaA (A) at the origin during replication. When DnaN (N) is released after replication, YabA binds DnaN, releasing DnaA.
Despite much study, the mechanism of YabA action remains elusive. YabA is not able to promote DnaA–ATP hydrolysis in vitro [112], however, it has been shown to affect the co-operative binding of DnaA to oriC [73], and it is capable of disrupting DnaA oligomerisation in vitro [112]. It is not clear, however, if this is its main mode of action in vivo. Two alternative models have been proposed. In the first, YabA tethers DnaA to DnaN at the replisome for most of the cell cycle (Figure 8B) [113], sequestering DnaA from the origin during ongoing rounds of replication. This model is consistent with the alternate localisations of DnaA in wild type and ΔyabA cells. In wild type cells, DnaA localizes at the origin in small cells (which are at early points in their cell cycle) and at mid-cell, co-incident with DnaX and therefore the replisome, in larger cells (in later stages of the cell cycle) [113]. In ΔyabA cells, by contrast, DnaA is localized with the origin throughout the cell cycle [113].
The alternative model proposes that YabA binds to DnaA at oriC so as to inhibit its cooperative binding to further DnaA molecules throughout the cell cycle up to the point where DNA replication is completed and the replisome disassembles. At this point free DnaN competitively titrates YabA away from its complex with DnaA, allowing the latter to bind cooperatively at the origin (Figure 8C) [73]. This model is consistent with evidence that the cellular level of DnaN correlates with the frequency of replication initiation, with increased DnaN levels increasing replication initiation frequency, and decreased levels, decreasing initiation frequency [73]. Additionally, in a strain replicating from a DnaA-independent origin, oriN, YabA was shown to affect the cooperativity of DnaA binding at oriC, and increased levels of DnaN removed YabA from oriC, suggesting that DnaN could be controlling the binding of YabA at the origin.
Further studies to establish the dynamics and stoichiometry of the interactions between YabA, DnaA and DnaN are required to further refine and reconcile these models: bearing in mind that they are unlikely to be mutually exclusive.
Soj/Spo0J
Soj is an ATPase which negatively and positively regulates DNA replication in B. subtilis [114], according to its oligomeric state [115], which is controlled by nucleotide binding. ATP-bound Soj forms dimers which co-operatively interact with DNA in a sequence unspecific manner, whilst ADP-bound Soj is monomeric [116]. Dimeric ATP-bound Soj appears to stimulate initiation of replication, whilst monomeric Soj inhibits replication [72,115]. Spo0J regulates Soj activity by stimulating its ATPase activity, thus converting the dimer back to the monomeric form [115].
Soj appears to interact with the ATPase domain (III) of DnaA, although it does not affect ATP binding or hydrolysis by DnaA [72]. Instead, it acts by inhibiting DnaA oligomerisation at oriC. A Soj mutant trapped in the monomeric state has been shown to inhibit DnaA oligomer formation both in vitro and in vivo [72]. Curiously, Soj trapped in the dimeric state is also able to interact with DnaA on a similar surface, without inhibiting DnaA oligomerization. Thus, it has been suggested that monomeric Soj inhibits conformational changes in DnaA that are needed to form an active initiation complex [72], whilst dimeric Soj may stabilize DnaA in this oligomerization-competent conformation [72].
Soj and Spo0J are orthologues of ParA and ParB, respectively. ParA, and ParB, along with a cis-acting DNA sequence parS, are components of a plasmid partitioning system found in many prokaryotic species. These systems ensure partitioning of low copy number plasmids into daughter cells. ParB binds to parS sequences on the plasmid, while ParA forms filaments on chromosomal DNA. An interaction between ParA and ParB simulates the ATPase activity of the former, which is thought to cause dissociation of the terminal ParA molecule from the filament; the plasmid can then either dissociate or translocate along the chromosomal DNA by binding to the next ParA molecule. Continuous cycles of ParA assembly and disassembly lead to equidistribution of the plasmids within the cell [117], ensuring partitioning on either side of the division plane [118].
Chromosomal orthologues of ParA and ParB and parS sites are found in some bacterial species and it is attractive to assume that they perform a role in chromosomal segregation similar to that of the plasmid partitioning proteins. In B. subtilis, although Spo0J-parS contributes to accurate chromosome segregation, it is not essential for this function [119]. Instead it plays a role in the recruitment of the SMC complex to the origin, and it is the SMC proteins that are responsible for proper segregation and condensation of the chromosome [120,121]. Regardless, Spo0J provides a mechanism through which B. subtilis may be able to co-ordinate DNA replication and chromosome segregation [121].
DnaD
DnaD has also been reported to play a role in the regulation of DNA replication initiation in B. subtilis. Like YabA, DnaD has been shown to inhibit the ATP-dependent cooperative binding of DnaA to oriC DNA [122] and to affect the formation of helical DnaA filaments in vitro [112]. It remains unclear however, how these activities can be reconciled with the role of DnaD in vivo, where it is essential for DNA replication initiation.
DnaA-Box Clusters
A B. subtilis deletion strain, in which six DnaA-box clusters (DBCs) found outside of the replication origin were removed, displayed an early initiation of DNA replication phenotype. This phenotype was strong only when all six clusters were removed and could be partially relieved by the re-introduction of a single DBC at various locations [123]. Nevertheless, these data suggest that B. subtilis DNA replication is sensitive to the amount of free DnaA in the cell, which might otherwise be bound at these sites.
3.5.2. During Sporulation in Bacillus subtilis
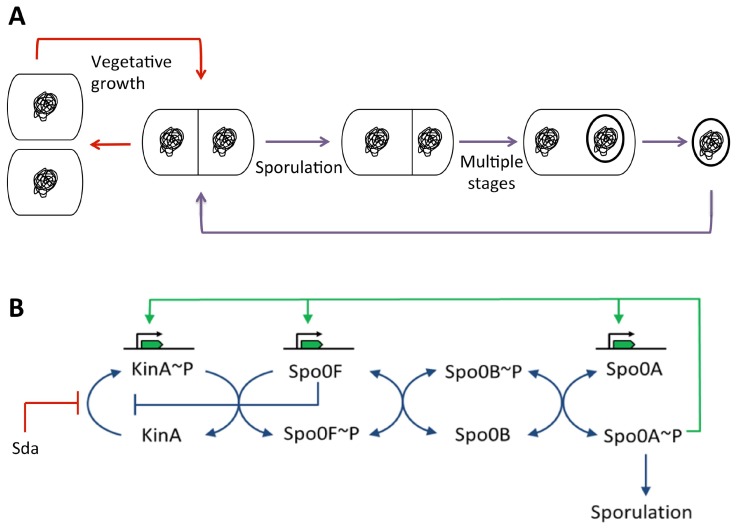
A characteristic of B. subtilis is its ability to differentiate under nutrient limiting conditions to form a dormant endospore. The spore is metabolically inactive and resistant to harsh conditions such as high temperatures, desiccation and ionizing radiation. When nutrients become available again, the spore can germinate, returning the cell to vegetative growth, even after thousands of years [124,125]. Unlike vegetative growth which is characterized by division at mid-cell, during sporulation the cell divides asymmetrically forming a larger mother cell compartment and smaller forespore compartment (Figure 9A). These two daughter cells each contain an identical copy of the genome, however, differential pathways of gene expression lead to dramatically different cell fates. The forespore is engulfed by the mother cell, and in the cytoplasm of the latter it matures into a resistant spore. In the final stages, the mother cell lyses to release the fully formed spore [126] (Figure 9A). Entry into the sporulation pathway is under the control of a complex signaling pathway, at the heart of which is an expanded two-component system termed the phosphorelay, which culminates in the phosphorylation of the response regulator Spo0A, the master control element of sporulation [127] (Figure 9B). Spo0A~P acts as a transcriptional regulator, controlling directly or indirectly the expression of over 500 genes [128].
Figure 9.
(A) Vegetative growth and sporulation in B. subtilis. In normal vegetative growth (red arrows) cells divide symmetrically, producing identical daughter cells. During sporulation (purple arrows) cells divide asymmetrically forming a mother cell and forespore; each receives an identical copy of the genome, and through differential gene regulation they experience different fates. The mother cell engulfs the forespore, nurturing it as it matures. In the final stages, the mother cell lyses releasing the dormant spore; (B) Phosphorelay leading to the induction of sporulation. A series of phosphoryl transfer reactions lead to the accumulation of threshold levels of Spo0A~P needed for entry into the sporulation pathway.
At the point of entry into sporulation, DNA replication and asymmetric cell division must be coordinated to ensure that the cell contains two, and only two, copies of the chromosome—one destined for the mother cell and the other for the forespore. Trapping of more than a single chromosome in the forespore compartment can reduce the viability of the spore and its capacity to germinate [129].
Spo0A~P Pulsing
It has long been recognized that there is a ‘sensitive period’ in the cell cycle when the cell can enter into the sporulation pathway. If the cell progresses beyond this point, it is committed to a new round of vegetative division [130,131]. The critical determinant is the concentration of Spo0A~P which fluctuates over the course of the cell cycle and is at its highest immediately after DNA replication is completed [129,132]. A threshold level of Spo0A~P must be reached for sporulation to be triggered. As Spo0A~P levels increase, low-threshold target genes are turned on, however, a higher threshold Spo0A~P concentration must be achieved in order to trigger the sporulation process [133,134].
Spo0A~P pulsing is linked to DNA replication, but until recently it was not known how. The cellular Spo0A~P concentration is controlled by the sporulation inhibitor protein, Sda [129]. Sda inhibits the sporulation sensor kinases KinA and KinB, which feed phosphate into the phosphorelay leading ultimately to the phosphorylation of Spo0A [135,136,137] (Figure 9B). Sda production is controlled at the transcriptional level by DnaA [129,135] such that sda expression requires the presence of replication active DnaA. Thus, Sda levels spike at the same time as, or just after, the replisome forms [129]. Sda is subsequently rapidly proteolysed [138]. This provides a feedback mechanism whereby Sda blocks phosphorylation of Spo0A and entry into sporulation, during ongoing rounds of DNA replication [129]. However, factors other than Sda influence Spo0A~P pulsing, as deletion of Sda does not prevent the pulsing of Spo0F levels (spo0F is a ‘low-threshold’ gene under the control of Spo0A~P) [139] suggesting that Spo0A~P pulsing still occurs.
The chromosomal arrangement of the phosphorelay genes spo0F and kinA is important for Spo0A~P pulsing [129]. spo0F is located close to the replication origin, in contrast to kinA which is located near the replication terminus. As a result, two copies of spo0F will be present in the cell during most of the period of DNA replication, alongside a single copy of kinA [132]. Alterations in the chromosomal positioning of spo0F, or induction of Spo0F from an inducible promoter, have been shown to affect Spo0A~P pulsing [132], with high Spo0F:KinA ratios inhibiting KinA phosphorylation and preventing sporulation [140,141]. As rapidly growing cells undertake multiple rounds of DNA replication simultaneously, the Spo0F:KinA ratio also provides a mechanism for inhibiting sporulation under nutrient-rich conditions. Collectively, the chromosomal arrangement of the phosphorelay genes in B. subtilis, together with direct inhibition of KinA activity by Sda, serve to coordinate the entry into sporulation with DNA replication.
SirA
Spo0A~P pulsing provides a mechanism for preventing replicating cells from entering into sporulation. Interestingly, cells which are artificially induced to sporulate under conditions of rapid growth are able to maintain correct chromosome copy number [142]. This is attributable to the activity of SirA, an inhibitor of DNA replication, produced under Spo0A~P control. Deletion of sirA results in loss of chromosome number control upon induction of sporulation during rapid growth [142]. Meanwhile, cells overproducing SirA do not form colonies on plates and in liquid culture many of these cells are elongated and anucleate, with some containing nucleoids which have been severed by division septa—a phenotype reminiscent of DnaA depletion [142,143]. SirA inhibits DNA replication through a direct interaction with DnaA [143]. A genetic screen indicated that the determinants of SirA binding reside in domain I of DnaA [54] and a later structure of a complex of SirA with DnaA domain I fully delineated this binding surface [144]. Cells harbouring alleles with sirA point mutations mapping to the DnaA domain I binding surface of SirA, exhibit a similar phenotype to ΔsirA cells. Moreover, these mutations disrupted SirA foci normally observed in sporulating cells [144]. This suggests that SirA localizes to the replisome via an interaction with DnaA during sporulation.
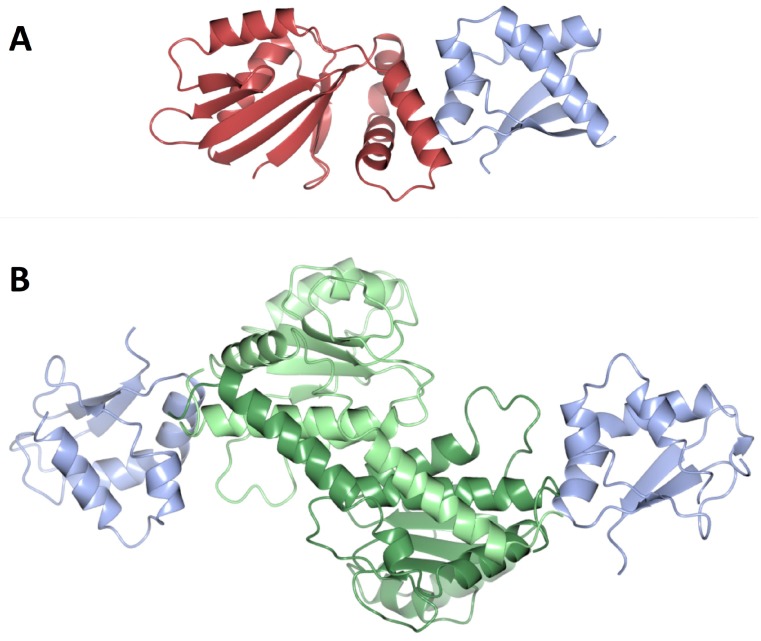
Intriguingly, SirA binds to a surface on DnaA domain I structurally equivalent to that used by the positive regulators of DNA replication initiation, DiaA and HobA, structural homologues found in E. coli and Helicobacter pylori, respectively (Figure 10). This raises an intriguing question about the role of DnaA domain I in replication initiation in the respective organisms. How is the same topological site used to positively and negatively regulate replication initiation?
Figure 10.
(A) SirA–DnaA-DomainI structure (PDB code: 4TPS). SirA is shown in crimson, domain I of DnaA in blue; (B) HobA–DnaA-DomainI structure (PDB code: 2WP0). HobA is shown in green, domain I of DnaA in blue. HobA and SirA interact on equivalent surfaces of DnaA, despite exerting different regulatory effects.
Recently, SirA was shown to facilitate chromosome segregation during sporulation, independent of its role in DNA replication regulation [145]. Newly synthesized bacterial origins are localized to a cell pole (or future pole) with high fidelity. In sporulating cells, oriC must be segregated or ‘captured’ at the respective poles in the future forespore and mother cell compartments following the onset of DNA replication. In a ΔsirA mutant strain, 10% of sporulating cells fail to capture oriC. This activity is distinct from the role of SirA in DNA replication regulation, as sirA mutants deficient in DNA replication inhibition were able to facilitate normal oriC capture. Soj has also been implicated in oriC capture during sporulation, with 20% of cells failing to capture oriC in a Δsoj mutant strain [120,145]. There is no further increase in the failure of cells to capture oriC in a ΔsojΔsirA double mutant, implying the two proteins are acting in the same pathway. Using a gain of interaction bacterial-two-hybrid screen, a potential interaction site between the C-terminus of SirA and domain III of DnaA was identified. This site overlaps with residues previously identified in the Soj-interaction site on DnaA, suggesting this interaction may facilitate oriC capture [145].
A Direct Role for Spo0A~P
The B. subtilis replication origin contains a number of Spo0A-boxes which partially overlap with DnaA-boxes [146] (Figure 4A). Indeed, the consensus Spo0A-box sequence 5′-TGTCGAA-3′ is similar to the DnaA-box consensus sequence 5’-TGTGNATAA-3’ [147]. Spo0A~P has been shown to bind these Spo0A-boxes in vitro [147], and sequence changes that alter the resemblance to the Spo0A-box consensus, without affecting that to the DnaA-box consensus, affect Spo0A~P, but not DnaA binding to the origin [146]. The binding of Spo0A~P to oriC appears to play a role in chromosome copy number control in a Δsda/ΔsirA mutant strain when sporulation is induced by starvation, or in cells induced to sporulate during rapid growth [146]. Δsda and ΔsirA strains each show a more significant loss of copy number control than upon mutation of the Spo0A boxes, with the phenotype being more profound for Δsda than ΔsirA [146].
4. In E. coli
4.1. Regulatory Inactivation of DnaA (RIDA)
In E. coli, the concentration of available ‘initiation-active’ DnaA–ATP is considered to be a limiting factor in the initiation of DNA replication from oriC [148]. Thus regulation of the availability of DnaA–ATP serves to control initiation events. The ‘regulatory inactivation of DnaA’ is a term given to the process of converting ‘active’ DnaA–ATP into ‘inactive’ DnaA–ADP by ATP hydrolysis. Hydrolysis is promoted by the protein Hda, and requires a complex between DnaA, Hda and the DNA-bound polymerase β-clamp, DnaN [149,150,151]. In this way, the regulation of initiation is coupled to elongation in DNA replication, as ATP hydrolysis becomes activated following the start of DNA synthesis [150].
Hda is homologous to DnaA domain III, with a 48% sequence similarity between their AAA+ ATPase domains [150]. Hda binds DnaN via an N-terminal clamp binding motif of sequence QL[SP]LPL [152], whilst Hda:DnaA interactions are mediated via their respective ATPase domains [153]. Strains carrying hda deletions, and inactivated Hda mutants or DnaA mutants unable to hydrolyse ATP, exhibit overinitiation of DNA replication and growth inhibition [48,148,149,154]. Hda-mediated hydrolysis of DnaA–ATP to DnaA–ADP requires ADP-bound Hda [155] which is monomeric. In contrast, apo-Hda appears to form homodimers and larger multimers [155] implying that Hda’s oligomerisation state plays a role in its ability to promote DnaA–ATP hydrolysis [155]. A crystal structure of Shewanella amazonensis Hda bound to the nucleotide CDP also revealed a dimer, however because the DnaN binding motif was buried, it was assumed to represent an inactive conformation of the protein [156].
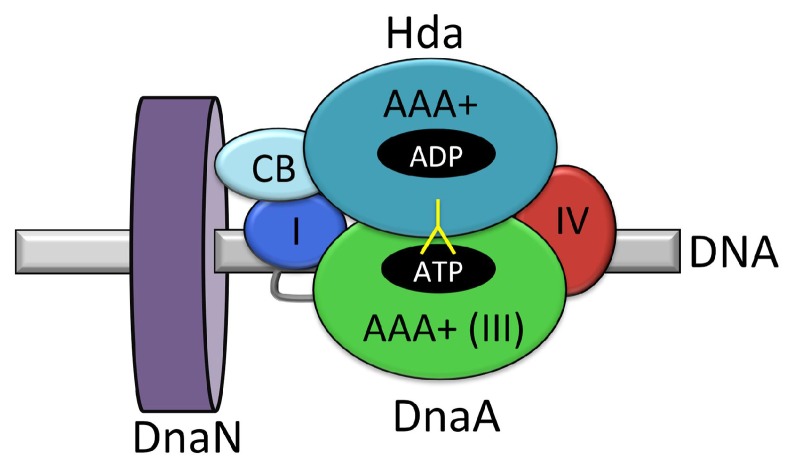
Mutations in both Hda and DnaA suggest that the proteins interact via their respective AAA+ domains, with the arginine finger residue of Hda playing an important role in DnaA–ATP hydrolysis [153,157]. Models of the DnaA–Hda interaction suggest that it may be similar to that formed between molecules of DnaA [156,157]. Hda’s interaction with the β-clamp is important for Hda–DnaA binding, suggesting that the β-clamp alters the conformation of the Hda–DnaA interaction to promote ATP hydrolysis [157]. Recently interactions of Hda with DnaA domains I and IV have also been shown to be important for RIDA as mutations at specific DnaA domain I and IV residues lead to higher cellular concentrations of DnaA–ATP than seen in wildtype cells [77,158]. The Hda–DnaA model suggests domain IV makes contacts with Hda at a nucleotide interaction surface towards the C-terminus of Hda [158]. It has therefore been proposed that DnaA domain I interacts with the N-terminal portion of Hda’s ATPase domain, to stabilize the DnaA–Hda interaction from both sides [77] (Figure 11). Further studies of the interactions between Hda, DnaA and the β-clamp are required to fully elucidate molecular mechanism of Hda action.
Figure 11.
Schematic representation of the interaction of Hda with DnaA and the β-clamp. Hda-ADP (light and dark cyan) makes contacts with domain I (blue) of DnaA–ATP principally through its clamp binding domain (CB), and with domains III (green) and IV (red) of DnaA through its AAA+ domain. An arginine finger from Hda (yellow) projects into the ATP-binding pocket of DnaA and facilitates ATP hydrolysis as part of the regulatory inactivation of DnaA (RIDA). The DNA-bound β-clamp is shown in purple. Figure adapted from [77].
The capacity of Hda to interact with DnaA and DnaN is functionally reminiscent of the B. subtilis regulator YabA, which also appears to couple the initiation and elongation steps in DNA replication. However, the mechanism of action of the two proteins is quite different. The proteins are structurally and mechanistically distinct. Hda influences DnaA–ATP hydrolysis in E. coli, while YabA has no effect on the hydrolysis of DnaA–ATP in B. subtilis. The latter instead appears to influence the oligomerisation of DnaA at the origin.
4.2. IHF and Fis
The DNA-bending proteins integration host factor (IHF) and Fis are thought to play important roles in regulating the binding of DnaA at the origin of replication [159]. Specifically, they have been shown to shape the binding of DnaA to two cis-acting regulatory sites on the chromosome, datA and DARS [160,161] (see Section 4.3 below). Loss of IHF disrupts synchronous DNA replication. Curiously, Fis has been reported to play both inhibitory and stimulatory roles in DNA replication initiation [162,163,164].
Both proteins bind to oriC and act in an antagonistic manner [159,165]. Binding of IHF to a specific site in the E. coli replication origin as shown in Figure 4B promotes binding of DnaA at DnaA-boxes within the origin [166], contributing to DnaA oligomer formation. The binding of IHF induces a bend in the DNA, which is proposed to bring the two adjacent DnaA-boxes into closer proximity, facilitating the extension of the helical DnaA oligomer [65]. Fis has been reported to inhibit DNA unwinding at oriC by blocking binding of both DnaA and IHF [159]. Increasing concentrations of DnaA were found to relieve Fis inhibition, and IHF was found to redistribute DnaA molecules at oriC [159,166].
4.3. DnaA-Box Sequences: datA and DARS
In stark contrast to the clusters of DnaA-box sequences of B. subtilis, which do not play a significant role in replication initiation, E. coli possesses three loci with DnaA-box motifs that are used to regulate DNA replication initiation.
One such locus, datA, ≈1 kb in length and located at 94.7 min on the E. coli chromosome [167], contains five DnaA-boxes with high affinity for DnaA. It acts as a negative regulator of DNA replication initiation; deletion of datA or mutations of the DnaA-boxes within datA causes over-initiation of DNA replication [167,168,169]. Binding of IHF to datA promotes DnaA-binding and is essential for the regulatory action of datA [170]. datA had been suggested to act as a sink titrating DnaA away from the replication origin [167,168,169,170]. A recent study however, has revealed that datA promotes the hydrolysis of DnaA–ATP to DnaA–ADP [161], in a manner that is dependent on both IHF binding to datA and the DnaA arginine finger residue (Arg285). This implies that datA promotes the formation of a nucleoprotein complex, somewhat reminiscent of that formed at oriC, and stimulates the hydrolysis of DnaA–ATP at this site [161]. The binding of IHF to datA takes place immediately after initiation, providing a mechanism for the timing of datA mediated DnaA–ATP hydrolysis [161].
Two other DnaA-binding loci in E. coli, termed DARS1 and DARS2 for DnaA reactivating site 1 and 2, respectively, have been implicated in the reactivation of DnaA by promoting nucleotide exchange, generating DnaA–ATP from DnaA–ADP [171]. Located at 17.5 min and 64 min on the chromosome, respectively, deletion of DARS sequences causes inhibition of DNA replication due to a decrease in the cellular DnaA–ATP concentration [171]. DnaA–ADP molecules have been shown to assemble on DARS1 promoting the regeneration of DnaA–ATP [171]. The simultaneous binding of the DNA bending proteins IHF and Fis to DARS2 has been shown to facilitate DnaA–ATP regeneration in vivo [160], providing a mechanism for the timing of DnaA reactivation. The binding of IHF to DARS2 appears to be cell-cycle regulated and independent of DNA replication, whilst the binding of Fis is linked to growth phase: occurring during exponential growth but not stationary phase [160]. The role of Fis at DARS2 is consistent with a report that Fis is required for the stimulation of replication initiation in rapidly growing cells [164].
The chromosomal positioning of datA, DARS1 and, particularly, DARS2, relative to oriC has been shown to be important for the proper timing of DNA replication initiation. Translocation of these sites perturbs regulation of initiation [172,173]. However, relocation of datA and DARS1 perturbs DNA replication initiation only when they are moved in close proximity to the replication terminus or origin, respectively. In both cases, these effects could be attributed to a gene dosage effect, or decreased/increased proximity to DnaA [172]. The translocation of DARS2 however, has a more significant effect, with relocation of the site proximal to the terminus causing both decreased initiation events, and asynchronous replication. This suggests that the chromosomal location of DARS2 is important for regulating DNA replication synchrony [172,173].
4.4. SeqA
SeqA prevents re-initiation of DNA replication immediately after the previous round of replication has been initiated. It binds to hemimethylated GATC sites [174] in oriC and this serves to sequester the origin, preventing DnaA oligomer formation and transcription of the dnaA gene by blocking of the dnaA promoter [175,176,177,178]. The E. coli replication origin contains 11 GATC sites which are hemimethylated immediately after DNA replication has been initiated, because the newly synthesized strand is yet to be methylated whilst the parental DNA strand is methylated. SeqA bound to the hemimethylated GATC sites at oriC recruits further SeqA proteins. The origin is thus sequestered as long as six or more of the GATC sites are hemimethylated [179]. Sequestration of the origin persists for around a third of the cell cycle, after which time a combination of SeqA dissociation and methylation of the adenosine bases in the GATC sites of the newly synthesized strand by Dam methyltransferase relieves sequestration [177,180,181]. Interestingly, SeqA has also been implicated in faithful chromosome segregation [182].
4.5. DiaA (and HobA)
DiaA is a positive regulator of DNA replication initiation in E. coli influencing the frequency and timing of the initiation event [56]. It functions by binding to domain I of DnaA and promoting the oligomerisation of DnaA at the origin [82,183,184]. HobA, an orthologue of DiaA in H. pylori [185], is an essential regulator of DNA replication in this organism [55]. DiaA and HobA are tetramers. The structure of a HobA–DnaA domain I complex revealed a 4:4 stoichiometry, with each HobA protomer bound to one DnaA domain I [82]. DiaA has been shown to bind an equivalent site on DnaA [76], although HobA and DiaA are not interchangeable in vivo due to differences in their cognate DnaA domain I sequences [183]. Heterologous complexes can be achieved however with hybrid DnaA molecules. Thus DiaA from E. coli can interact with a chimaeric protein resulting from fusion of DnaA domain I from E. coli and DnaA domains II-IV from H. pylori. This confirms that DiaA and HobA are functional homologs, each promoting DnaA binding at the origin, albeit with different dynamics. HobA accelerates DnaA binding, whilst DiaA decreases the DnaA binding rate [183]. Structural and mutational studies with HobA have led to the suggestion that the tetramers function as molecular scaffolds which promote the formation of DnaA oligomers at oriC, however direct experimental evidence is still required [186]. DiaA may play a role in regulating the timing of helicase loading in E. coli as both proteins appear to bind to an overlapping site on DnaA, and DiaA has been shown to inhibit helicase loading in vitro [76].
4.6. Lysine Acetylation of DnaA
A recently discovered mechanism for controlling DNA replication initiation in E. coli involves the reversible acetylation of lysines within DnaA. Acetylation sites were identified on 13 lysines within natively expressed DnaA, including a key lysine (Lys178) required for the binding of ATP [187]. The acetylation of this residue was growth phase dependent, with peak levels observed in stationary phase. Mutation of Lys178 to Gln or Arg prevented ATP-binding to DnaA, suggesting acetylation of Lys178 would have a similar effect in inactivating the initiator. It is attractive to consider that similar, as yet unidentified, post-translational modifications may exist in other species providing an elegant mechanism for coupling DNA replication initiation to growth phase [187].
5. Regulatory Mechanisms for DNA Replication Regulation: B. subtilis vs. E. coli
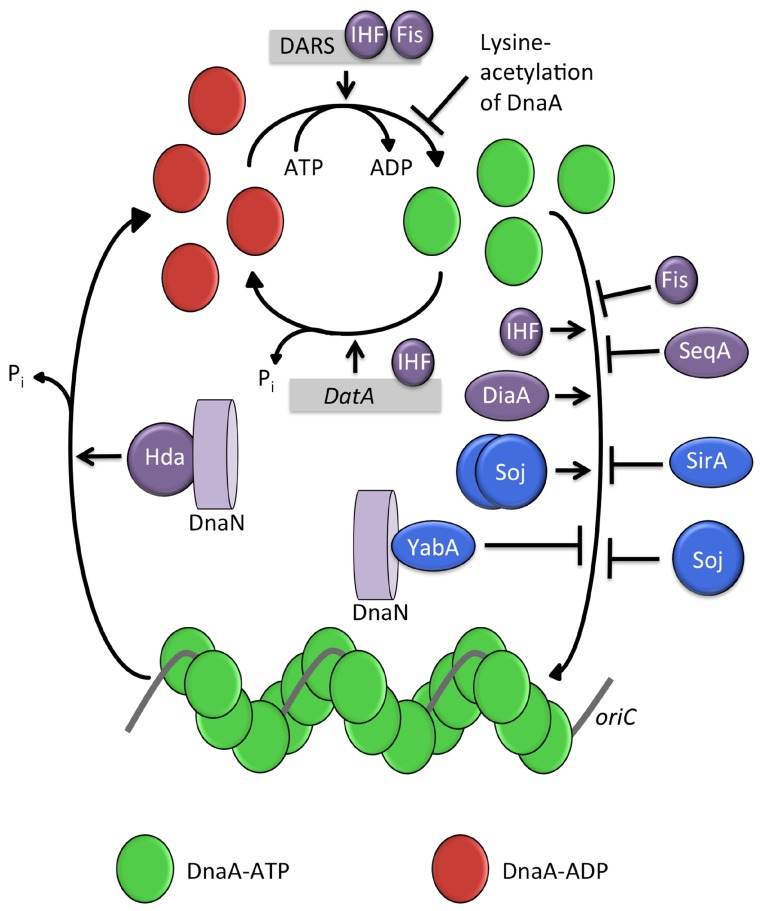
In recent years, opposing themes have emerged in the regulation of DNA replication initiation in E. coli and B. subtilis (Figure 12). It has long been recognized that the cellular DnaA–ATP concentration plays an important role in the regulation of E. coli replication initiation [1,4,188]. Many of the regulatory mechanisms in E. coli have been found to influence the adenosine nucleotide bound state of DnaA. Hda, along with the DNA polymerase clamp, DnaN, acts to promote the hydrolysis of DnaA–ATP after initiation [150]. Meanwhile, the datA, DARS1 and DARS2 loci influence available DnaA–ATP levels by promoting ATP-hydrolysis (datA) [161] or nucleotide exchange from ADP to ATP (DARS) [171]. The DNA-binding proteins Fis and IHF influence DnaA binding at these sites so as to control the cellular DnaA–ATP concentration [159,160]. Finally, lysine acetylation of DnaA coordinated with growth cycle is also believed to affect ATP-binding to DnaA and thus inhibit initiation of DNA replication in later growth phases [187].
Figure 12.
Schematic representation of the mechanisms regulating DNA replication initiation in E. coli and B. subtilis. Key regulators in E. coli influence the adenosine nucleotide bound state of DnaA, whilst those in B. subtilis influence the binding of DnaA–ATP to oriC. For E. coli, the protein regulators are shown in purple, and the DNA binding sites, DatA and DARS, are shown in grey. For B. subtilis, protein regulators are shown in blue. Pointed arrows indicate positive effects upon a process, and blunt ended arrows indicate inhibitory effects.
In B. subtilis, by contrast, no regulator has been identified which affects the conversion of DnaA–ATP to DnaA–ADP. Instead, replication regulators in B. subtilis appear to act directly on the binding of DnaA to oriC. YabA is able to inhibit DnaA oligomer formation in vitro [112] and to affect the cooperativity of DnaA–ATP binding at oriC [73,122]. Monomeric Soj appears directly to inhibit DnaA oligomer formation on DNA, whilst dimeric Soj seems to be able to promote this oligomerisation [72]. Despite the fact that Soj and YabA interact with the ATPase domain of DnaA, neither protein has an effect on ATP hydrolysis in DnaA–ATP [72,112]. Instead both appear to target the DnaA oligomerisation determinants residing in domain III. Furthermore, DnaA-box clusters with significant roles in the regulation of DNA replication initiation have not been identified in B. subtilis [123], in marked contrast to E. coli. Together this evidence suggests that the primary mechanisms of DNA replication control are different in B. subtilis and E. coli. The initiation regulators determine the ligation status (ATP versus ADP) of DnaA in E. coli while in B. subtilis they act to control the downstream event of DnaA oligomerisation at oriC.
Despite this, a number of parallels can be drawn between the regulatory mechanisms in the two species. Both organisms utilize a major regulator during vegetative growth which interacts with both DnaA and DnaN; YabA in B. subtilis [110] and Hda in E. coli [152]. This may provide the respective species with a mechanism for appropriately timing initiation of replication, since DnaN is a key component of the DNA elongation complex. Both organisms have regulators which are implicated in chromosome segregation, SeqA in E. coli, and Soj and SirA during growth and sporulation of B. subtilis, respectively. Both organisms appear to utilize a method of origin sequestration to prevent DnaA binding: in E. coli, SeqA binds to newly replicated origins, and in B. subtilis Spo0A~P is able to bind to the origin, playing an albeit more modest role in inhibiting DNA replication. Furthermore, both organisms have evolved a regulator which targets a structurally equivalent location on DnaA domain I—the sporulation inhibitor of replication in B. subtilis, SirA, and the promoter of DNA replication initiation in E. coli, DiaA. Thus, these may represent common themes of replication regulation across bacterial species.
Acknowledgments
K.H.J. was the recipient of a DTA Studentship awarded by the Biotechnology and Biological Sciences Research Council, UK. Work on DNA replication proteins at York was supported by the European Integrated Project, BaSysBio LSHG-CT-2006-037469.
Author Contributions
K.H.J. reviewed the literature and prepared the first draft of this review as part of studies towards her PhD which was supervised by A.J.W.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kaguni J.M. Replication initiation at the Escherichia coli chromosomal origin. Curr. Opin. Chem. Biol. 2011;15:606–613. doi: 10.1016/j.cbpa.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katayama T., Ozaki S., Keyamura K., Fujimitsu K. Regulation of the replication cycle: Conserved and diverse regulatory systems for DnaA and oriC. Nat. Rev. Microbiol. 2010;8:163–170. doi: 10.1038/nrmicro2314. [DOI] [PubMed] [Google Scholar]
- 3.Scholefield G., Veening J.-W., Murray H. DnaA and ORC: More than DNA replication initiators. Trends Cell Biol. 2011;21:188–194. doi: 10.1016/j.tcb.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Mott M.L., Berger J.M. DNA replication initiation: Mechanisms and regulation in bacteria. Nat. Rev. Microbiol. 2007;5:343–354. doi: 10.1038/nrmicro1640. [DOI] [PubMed] [Google Scholar]
- 5.Leonard A.C., Grimwade J.E. Regulation of DnaA assembly and activity: Taking directions from the genome. Annu. Rev. Microbiol. 2011;65:19–35. doi: 10.1146/annurev-micro-090110-102934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briggs G.S., Smits W.K., Soultanas P. Chromosomal replication initiation machinery of low-G+C-content Firmicutes. J. Bacteriol. 2012;194:5162–5170. doi: 10.1128/JB.00865-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zorman S., Seitz H., Sclavi B., Strick T.R. Topological characterization of the DnaA-oriC complex using single-molecule nanomanipuation. Nucleic Acids Res. 2012;40:7375–7383. doi: 10.1093/nar/gks371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erzberger J.P., Mott M.L., Berger J.M. Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat. Struct. Mol. Biol. 2006;13:676–683. doi: 10.1038/nsmb1115. [DOI] [PubMed] [Google Scholar]
- 9.Fuller R.S., Funnell B.E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984;38:889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- 10.Kowalski D., Eddy M.J. The DNA unwinding element: A novel, cis-acting component that facilitates opening of the Escherichia coli replication origin. EMBO J. 1989;8:4335–4344. doi: 10.1002/j.1460-2075.1989.tb08620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988;52:743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- 12.Marszalek J., Kaguni J.M. DnaA protein directs the binding of DnaB protein in initiation of DNA replication in Escherichia coli. J. Biol. Chem. 1994;269:4883–4890. [PubMed] [Google Scholar]
- 13.Koboris J.A., Kornberg A. Escherichia coli dnaC Gene Product. Biol. Chem. 1982;257:13770–13775. [PubMed] [Google Scholar]
- 14.Fang L., Davey M.J., O’Donnell M. Replisome assembly at oriC, the replication origin of E. coli, reveals an explanation for initiation sites outside an origin. Mol. Cell. 1999;4:541–553. doi: 10.1016/S1097-2765(00)80205-1. [DOI] [PubMed] [Google Scholar]
- 15.Bruand C., Ehrlich S.D., Jannière L. Primosome assembly site in Bacillus subtilis. EMBO J. 1995;14:2642–2650. doi: 10.1002/j.1460-2075.1995.tb07262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W., Carneiro M.J.V.M., Turner I.J., Allen S., Roberts C.J., Soultanas P. The Bacillus subtilis DnaD and DnaB proteins exhibit different DNA remodelling activities. J. Mol. Biol. 2005;351:66–75. doi: 10.1016/j.jmb.2005.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smits W.K., Goranov A.I., Grossman A.D. Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo. Mol. Microbiol. 2010;75:452–461. doi: 10.1111/j.1365-2958.2009.06999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey S., Eliason W.K., Steitz T.A. Structure of hexameric DnaB helicase and its complex with a domain of DnaG primase. Science. 2007;318:459–463. doi: 10.1126/science.1147353. [DOI] [PubMed] [Google Scholar]
- 19.Reyes-Lamothe R., Sherratt D.J., Leake M.C. Stoichiometry and architecture of active DNA replication machinery in Escherichia coli. Science. 2010;328:498–501. doi: 10.1126/science.1185757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson A., Causer R.J., Dixon N.E. Architecture and Conservation of the Bacterial DNA Replication Machinery, an Underexploited Drug Target. Curr. Drug Targets. 2012;13:352–372. doi: 10.2174/138945012799424598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beattie T.R., Reyes-Lamothe R. A Replisome’s journey through the bacterial chromosome. Front. Microbiol. 2015;6:1–12. doi: 10.3389/fmicb.2015.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voet D., Voet J.G. Biochemistry. Wiley; Hoboken, NJ, USA: 2011. DNA Replication, Repair, and Recombination; pp. 1171–1259. [Google Scholar]
- 23.Corn J.E., Pelton J.G., Berger J.M. Identification of a DNA primase template tracking site redefines the geometry of primer synthesis. Nat. Struct. Mol. Biol. 2008;15:163–169. doi: 10.1038/nsmb.1373. [DOI] [PubMed] [Google Scholar]
- 24.Corn J.E., Berger J.M. Regulation of bacterial priming and daughter strand synthesis through helicase-primase interactions. Nucleic Acids Res. 2006;34:4082–4088. doi: 10.1093/nar/gkl363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corn J.E., Pease P.J., Hura G.L., Berger J.M. Crosstalk between primase subunits can act to regulate primer synthesis in trans. Mol. Cell. 2005;20:391–401. doi: 10.1016/j.molcel.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Dervyn E., Suski C., Daniel R., Bruand C., Chapuis J., Errington J., Jannière L., Ehrlich S.D. Two essential DNA polymerases at the bacterial replication fork. Science. 2001;294:1716–1719. doi: 10.1126/science.1066351. [DOI] [PubMed] [Google Scholar]
- 27.Sanders G.M., Dallmann H.G., McHenry C.S. Reconstitution of the B. subtilis Replisome with 13 Proteins Including Two Distinct Replicases. Mol. Cell. 2010;37:273–281. doi: 10.1016/j.molcel.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Hill T.M., Henson J.M., Kuempel P.L. The terminus region of the Escherichia coli chromosome contains two separate loci that exhibit polar inhibition of replication. Proc. Natl. Acad. Sci. USA. 1987;84:1754–1758. doi: 10.1073/pnas.84.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidaka M., Kobayashi T., Takenaka S., Takeya H., Horiuchi T. Purification of a DNA replication terminus (ter) site-binding protein in Escherichia coli and identification of the structural gene. J. Biol. Chem. 1989;264:21031–21037. [PubMed] [Google Scholar]
- 30.Neylon C., Kralicek A.V., Hill T.M., Dixon N.E. Replication Termination in Escherichia coli: Structure and Antihelicase Activity of the Tus-Ter Complex. Microbiol. Mol. Biol. Rev. 2005;69:501–526. doi: 10.1128/MMBR.69.3.501-526.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamada K., Horiuchi T., Ohsumi K., Shimamoto N., Morikawa K. Structure of a replication-terminator protein complexed with DNA. Nature. 1996;383:598–603. doi: 10.1038/383598a0. [DOI] [PubMed] [Google Scholar]
- 32.Mulcair M.D., Schaeffer P.M., Oakley A.J., Cross H.F., Neylon C., Hill T.M., Dixon N.E. A Molecular Mousetrap Determines Polarity of Termination of DNA Replication in E. coli. Cell. 2006;125:1309–1319. doi: 10.1016/j.cell.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 33.Berghuis B.A., Dulin D., Xu Z.-Q., van Laar T., Cross B., Janissen R., Jergic S., Dixon N.E., Depken M., Dekker N.H. Strand separation establishes a sustained lock at the Tus–Ter replication fork barrier. Nat. Chem. Biol. 2015;11:579–585. doi: 10.1038/nchembio.1857. [DOI] [PubMed] [Google Scholar]
- 34.McNicholas S., Potterton E., Wilson K.S., Noble M.E.M. Presenting your structures: the CCP4mg molecular-graphics software. Acta. Cryst. D Biol. Crystallogr. 2011;67:386–394. doi: 10.1107/S0907444911007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill T.M. Arrest of bacterial DNA replication. Annu. Rev. Microbiol. 1992;46:603–633. doi: 10.1146/annurev.mi.46.100192.003131. [DOI] [PubMed] [Google Scholar]
- 36.Lewis P.J., Ralston G.B., Christopherson R.I., Wake R.G. Identification of the replication terminator protein binding sites in the terminus region of the Bacillus subtilis chromosome and stoichiometry of the binding. J. Mol. Biol. 1990;214:73–84. doi: 10.1016/0022-2836(90)90147-E. [DOI] [PubMed] [Google Scholar]
- 37.Vivian J.P., Porter C.J., Wilce J.A., Wilce M.C.J. An Asymmetric Structure of the Bacillus subtilis Replication Terminator Protein in Complex with DNA. J. Mol. Biol. 2007;370:481–491. doi: 10.1016/j.jmb.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 38.Langley D.B., Smith M.T., Lewis P.J., Wake R.G. Protein-nucleoside contacts in the interaction between the replication terminator protein of Bacillus subtilis and the DNA terminator. Mol. Microbiol. 1993;10:771–779. doi: 10.1111/j.1365-2958.1993.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 39.Wolanski M., Donczew R., Zawilak-Pawlik A., Zakrzewska-Czerwinska J. oriC-encoded instructions for the initiation of bacterial chromosome replication. Front. Microbiol. 2015;5:1–14. doi: 10.3389/fmicb.2014.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leonard A.C., Méchali M. DNA replication origins. Cold Spring Harb. Perspect. Biol. 2013;5:a010116. doi: 10.1101/cshperspect.a010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogasawara N., Yoshikawa H. Genes and their organization in the replication origin region of the bacterial chromosome. Mol. Microbiol. 1992;6:629–634. doi: 10.1111/j.1365-2958.1992.tb01510.x. [DOI] [PubMed] [Google Scholar]
- 42.Moriya S., Atlung T., Hansen F.G., Yoshikawa H., Ogasawara N. Cloning of an autonomously replicating sequence (ars) from the Bacillus subtilis chromosome. Mol. Microbiol. 1992;6:309–315. doi: 10.1111/j.1365-2958.1992.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 43.Donczew R., Weigel C., Lurz R., Zakrzewska-Czerwińska J., Zawilak-Pawlik A. Helicobacter pylori oriC-the first bipartite origin of chromosome replication in Gram-negative bacteria. Nucleic Acids Res. 2012;40:9647–9660. doi: 10.1093/nar/gks742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krause M., Rückert B., Lurz R., Messer W. Complexes at the replication origin of Bacillus subtilis with homologous and heterologous DnaA protein. J. Mol. Biol. 1997;274:365–380. doi: 10.1006/jmbi.1997.1404. [DOI] [PubMed] [Google Scholar]
- 45.Messer W., Blaesing F., Majka J., Nardmann J., Schaper S., Schmidt A., Seitz H., Speck C., Tüngler D., Wegrzyn G., et al. Functional domains of DnaA proteins. Biochimie. 1999;81:819–825. doi: 10.1016/S0300-9084(99)00215-1. [DOI] [PubMed] [Google Scholar]
- 46.Sutton M.D., Kaguni J.M. The Escherichia coli dnaA gene: Four functional domains. J. Mol. Biol. 1997;274:546–561. doi: 10.1006/jmbi.1997.1425. [DOI] [PubMed] [Google Scholar]
- 47.Fukuoka T., Moriya S., Yoshikawa H., Ogasawara N. Purification and characterization of an initiation protein for chromosomal replication, DnaA, in Bacillus subtilis. J. Biochem. 1990;107:732–739. doi: 10.1093/oxfordjournals.jbchem.a123117. [DOI] [PubMed] [Google Scholar]
- 48.Nishida S. A Nucleotide Switch in the Escherichia coli DnaA Protein Initiates Chromosomal Replication. J. Biol. Chem. 2002;277:14986–14995. doi: 10.1074/jbc.M108303200. [DOI] [PubMed] [Google Scholar]
- 49.Sekimizu K., Bramhill D., Kornberg A. ATP activates dnaA protein in initiating replication of plasmids bearing the origin of the E. coli chromosome. Cell. 1987;50:259–265. doi: 10.1016/0092-8674(87)90221-2. [DOI] [PubMed] [Google Scholar]
- 50.Roth A., Messer W. The DNA binding domain of the initiator protein DnaA. EMBO J. 1995;14:2106–2111. doi: 10.1002/j.1460-2075.1995.tb07202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujikawa N., Kurumizaka H., Nureki O., Terada T. Structural basis of replication origin recognition by the DnaA protein. Nucleic Acids Res. 2003;31:2077–2086. doi: 10.1093/nar/gkg309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaguni J.M. DnaA: Controlling the initiation of bacterial DNA replication and more. Annu. Rev. Microbiol. 2006;60:351–375. doi: 10.1146/annurev.micro.60.080805.142111. [DOI] [PubMed] [Google Scholar]
- 53.Nozaki S., Ogawa T. Determination of the minimum domain II size of Escherichia coli DnaA protein essential for cell viability. Microbiology. 2008;154:3379–3384. doi: 10.1099/mic.0.2008/019745-0. [DOI] [PubMed] [Google Scholar]
- 54.Rahn-Lee L., Merrikh H., Grossman A.D., Losick R. The sporulation protein SirA inhibits the binding of DnaA to the origin of replication by contacting a patch of clustered amino acids. J. Bacteriol. 2011;193:1302–1307. doi: 10.1128/JB.01390-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zawilak-Pawlik A., Kois A., Stingl K., Boneca I.G., Skrobuk P., Piotr J., Lurz R., Zakrzewska-Czerwińska J., Labigne A. HobA—A novel protein involved in initiation of chromosomal replication in Helicobacter pylori. Mol. Microbiol. 2007;65:979–994. doi: 10.1111/j.1365-2958.2007.05853.x. [DOI] [PubMed] [Google Scholar]
- 56.Ishida T., Akimitsu N., Kashioka T., Hatano M., Kubota T., Ogata Y., Sekimizu K., Katayama T. DiaA, a novel DnaA-binding protein, ensures the timely initiation of Escherichia coli chromosome replication. J. Biol. Chem. 2004;279:45546–45555. doi: 10.1074/jbc.M402762200. [DOI] [PubMed] [Google Scholar]
- 57.Seitz H., Weigel C., Messer W. The interaction domains of the DnaA and DnaB replication proteins of Escherichia coli. Mol. Microbiol. 2000;37:1270–1279. doi: 10.1046/j.1365-2958.2000.02096.x. [DOI] [PubMed] [Google Scholar]
- 58.Margulies C., Kaguni J.M. Ordered and sequential binding of DnaA protein to oriC, the chromosomal origin of Escherichia coli. J. Biol. Chem. 1996;271:17035–17040. doi: 10.1074/jbc.271.29.17035. [DOI] [PubMed] [Google Scholar]
- 59.Langer U., Richter S., Roth A., Weigel C., Messer W. A comprehensive set of DnaA-box mutations in the replication origin, oriC, of Escherichia coli. Mol. Microbiol. 1996;21:301–311. doi: 10.1046/j.1365-2958.1996.6481362.x. [DOI] [PubMed] [Google Scholar]
- 60.Schaper S., Messer W. Interaction of the initiator protein DnaA of Escherichia coli with its DNA target. J. Biol. Chem. 1995;270:17622–17626. doi: 10.1074/jbc.270.29.17622. [DOI] [PubMed] [Google Scholar]
- 61.Blaesing F., Weigel C., Welzeck M., Messer W. Analysis of the DNA-binding domain of Escherichia coli DnaA protein. Mol. Microbiol. 2000;36:557–569. doi: 10.1046/j.1365-2958.2000.01881.x. [DOI] [PubMed] [Google Scholar]
- 62.Miller D.T., Grimwade J.E., Betteridge T., Rozgaja T., Torgue J.J.-C., Leonard A.C. Bacterial origin recognition complexes direct assembly of higher-order DnaA oligomeric structures. Proc. Natl. Acad. Sci. USA. 2009;106:18479–18484. doi: 10.1073/pnas.0909472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rozgaja T.A., Grimwade J.E., Iqbal M., Czerwonka C., Vora M., Leonard A.C. Two oppositely oriented arrays of low affinity recognition sites in oriC guide progressive binding of DnaA during E. coli pre-RC assembly. Mol. Microbiol. 2012;82:475–488. doi: 10.1111/j.1365-2958.2011.07827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGarry K.C., Ryan V.T., Grimwade J.E., Leonard A.C. Two discriminatory binding sites in the Escherichia coli replication origin are required for DNA strand opening by initiator DnaA-ATP. Proc. Natl. Acad. Sci. USA. 2004;101:2811–2816. doi: 10.1073/pnas.0400340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaur G., Vora M.P., Czerwonka C.A., Rozgaja T.A., Grimwade J.E., Leonard A.C. Building the bacterial orisome: High affinity DnaA recognition plays a role in setting the conformation of oriC DNA. Mol. Microbiol. 2014;91:1148–1163. doi: 10.1111/mmi.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erzberger J.P., Pirruccello M.M., Berger J.M. The structure of bacterial DnaA: Implications for general mechanisms underlying DNA replication initiation. EMBO J. 2002;21:4763–4773. doi: 10.1093/emboj/cdf496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogura T., Whiteheart S.W., Wilkinson A.J. Conserved arginine residues implicated in ATP hydrolysis, nucleotide-sensing, and inter-subunit interactions in AAA and AAA+ ATPases. J. Struct. Biol. 2004;146:106–112. doi: 10.1016/j.jsb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 68.Duderstadt K.E., Mott M.L., Crisona N.J., Chuang K., Yang H., Berger J.M. Origin remodeling and opening in bacteria rely on distinct assembly states of the DnaA initiator. J. Biol. Chem. 2010;285:28229–28239. doi: 10.1074/jbc.M110.147975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duderstadt K.E., Chuang K., Berger J.M. DNA stretching by bacterial initiators promotes replication origin opening. Nature. 2011;478:209–213. doi: 10.1038/nature10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Speck C., Messer W. Mechanism of origin unwinding: Sequential binding of DnaA to double- and single-stranded DNA. EMBO J. 2001;20:1469–1476. doi: 10.1093/emboj/20.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richardson T.T., Harran O., Murray H. The bacterial DnaA-trio replication origin element specifies single-stranded DNA initiator binding. Nature. 2016;534:412–416. doi: 10.1038/nature17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scholefield G., Errington J., Murray H. Soj/ParA stalls DNA replication by inhibiting helix formation of the initiator protein DnaA. EMBO J. 2012;31:1–14. doi: 10.1038/emboj.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merrikh H., Grossman A.D. Control of the replication initiator DnaA by an anti-cooperativity factor. Mol. Microbiol. 2011;82:434–446. doi: 10.1111/j.1365-2958.2011.07821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rotoli S.M., Biswas-Fiss E., Biswas S.B. Quantitative analysis of the mechanism of DNA binding by Bacillus DnaA protein. Biochimie. 2012;94:2764–2775. doi: 10.1016/j.biochi.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 75.Sutton M.D., Carr K.M., Vicente M., Kaguni J.M. Escherichia coli DnaA Protein. J. Biol. Chem. 1998;273:34255–34262. doi: 10.1074/jbc.273.51.34255. [DOI] [PubMed] [Google Scholar]
- 76.Keyamura K., Abe Y., Higashi M., Ueda T., Katayama T. DiaA dynamics are coupled with changes in initial origin complexes leading to helicase loading. J. Biol. Chem. 2009;284:25038–25050. doi: 10.1074/jbc.M109.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su’etsugu M., Harada Y., Keyamura K., Matsunaga C., Kasho K., Abe Y., Ueda T., Katayama T. The DnaA N-terminal domain interacts with Hda to facilitate replicase clamp-mediated inactivation of DnaA. Environ. Microbiol. 2013;15:3183–3195. doi: 10.1111/1462-2920.12147. [DOI] [PubMed] [Google Scholar]
- 78.Carr K.M., Kaguni J.M. Stoichiometry of DnaA and DnaB protein in initiation at the Escherichia coli chromosomal origin. J. Biol. Chem. 2001;276:44919–44925. doi: 10.1074/jbc.M107463200. [DOI] [PubMed] [Google Scholar]
- 79.Weigel C., Seitz H. Strand-specific loading of DnaB helicase by DnaA to a substrate mimicking unwound oriC. Mol. Microbiol. 2002;46:1149–1156. doi: 10.1046/j.1365-2958.2002.03232.x. [DOI] [PubMed] [Google Scholar]
- 80.Felczak M.M., Simmons L.A., Kaguni J.M. An essential tryptophan of Escherichia coli DnaA protein functions in oligomerization at the E. coli replication origin. J. Biol. Chem. 2005;280:24627–24633. doi: 10.1074/jbc.M503684200. [DOI] [PubMed] [Google Scholar]
- 81.Simmons L.A., Felczak M., Kaguni J.M. DnaA Protein of Escherichia coli: Oligomerization at the E. coli chromosomal origin is required for initiation and involves specific N-terminal amino acids. Mol. Microbiol. 2003;49:849–858. doi: 10.1046/j.1365-2958.2003.03603.x. [DOI] [PubMed] [Google Scholar]
- 82.Natrajan G., Noirot-Gros M.-F., Zawilak-Pawlik A., Kapp U., Terradot L. The structure of a DnaA/HobA complex from Helicobacter pylori provides insight into regulation of DNA replication in bacteria. Proc. Natl. Acad. Sci. USA. 2009;106:21115–21120. doi: 10.1073/pnas.0908966106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lowery T.J., Pelton J.G., Chandonia J.-M., Kim R., Yokota H., Wemmer D.E. NMR structure of the N-terminal domain of the replication initiator protein DnaA. J. Struct. Funct. Genom. 2007;8:11–17. doi: 10.1007/s10969-007-9022-7. [DOI] [PubMed] [Google Scholar]
- 84.Abe Y., Jo T., Matsuda Y., Matsunaga C., Katayama T., Ueda T. Structure and function of DnaA N-terminal domains: Specific sites and mechanisms in inter-DnaA interaction and in DnaB helicase loading on oriC. J. Biol. Chem. 2007;282:17816–17827. doi: 10.1074/jbc.M701841200. [DOI] [PubMed] [Google Scholar]
- 85.Molt K.L., Sutera V.A., Moore K.K., Lovett S.T. A role for nonessential domain II of initiator protein, DnaA, in replication control. Genetics. 2009;183:39–49. doi: 10.1534/genetics.109.104760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arias-Palomo E., O’Shea V.L., Hood I.V., Berger J.M. The bacterial DnaC helicase loader is a DnaB ring breaker. Cell. 2013;153:438–448. doi: 10.1016/j.cell.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang W., Marszalek J., Zhang W., Hupp T.R., Margulies C., Carr K.M., Cherry S., Kaguni J.M. Domains of DnaA Protein Involved in Interaction with DnaB Protein, and in Unwinding the Escherichia coli Chromosomal Origin. J. Biol. Chem. 1996;271:18535–18542. doi: 10.1074/jbc.271.31.18535. [DOI] [PubMed] [Google Scholar]
- 88.Mott M.L., Erzberger J.P., Coons M.M., Berger J.M. Structural synergy and molecular crosstalk between bacterial helicase loaders and replication initiators. Cell. 2008;135:623–634. doi: 10.1016/j.cell.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Makowska-Grzyska M., Kaguni J.M. Primase Directs the Release of DnaC from DnaB. Mol. Cell. 2010;37:90–101. doi: 10.1016/j.molcel.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bell S.P., Kaguni J.M. Helicase loading at chromosomal origins of replication. Cold Spring Harb. Perspect. Biol. 2013;5:1–20. doi: 10.1101/cshperspect.a010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soultanas P. Loading mechanisms of ring helicases at replication origins. Mol. Microbiol. 2012;84:6–16. doi: 10.1111/j.1365-2958.2012.08012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Velten M., McGovern S., Marsin S., Ehrlich S.D., Noirot P., Polard P. A two-protein strategy for the functional loading of a cellular replicative DNA helicase. Mol. Cell. 2003;11:1009–1020. doi: 10.1016/S1097-2765(03)00130-8. [DOI] [PubMed] [Google Scholar]
- 93.Ioannou C., Schaeffer P.M., Dixon N.E., Soultanas P. Helicase binding to DnaI exposes a cryptic DNA-binding site during helicase loading in Bacillus subtilis. Nucleic Acids Res. 2006;34:5247–5258. doi: 10.1093/nar/gkl690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu B., Eliason W.K., Steitz T.A. Structure of a helicase-helicase loader complex reveals insights into the mechanism of bacterial primosome assembly. Nat. Commun. 2013;4:1–8. doi: 10.1038/ncomms3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoshino T., McKenzie T., Schmidt S., Tanaka T., Sueoka N. Nucleotide sequence of Bacillus subtilis dnaB: A gene essential for DNA replication initiation and membrane attachment. Proc. Natl. Acad. Sci. USA. 1987;84:653–657. doi: 10.1073/pnas.84.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marston F.Y., Grainger W.H., Smits W.K., Hopcroft N.H., Green M., Hounslow A.M., Grossman A.D., Craven C.J., Soultanas P. When simple sequence comparison fails: The cryptic case of the shared domains of the bacterial replication initiation proteins DnaB and DnaD. Nucleic Acids Res. 2010;38:6930–6942. doi: 10.1093/nar/gkq465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bruand C., Farache M., McGovern S., Ehrlich S.D., Polard P. DnaB, DnaD and DnaI proteins are components of the Bacillus subtilis replication restart primosome. Mol. Microbiol. 2001;42:245–255. doi: 10.1046/j.1365-2958.2001.02631.x. [DOI] [PubMed] [Google Scholar]
- 98.Ishigo-oka D., Ogasawara N., Moriya S. DnaD Protein of Bacillus subtilis Interacts with DnaA, the Initiator Protein of Replication. J. Bacteriol. 2001;183:1–4. doi: 10.1128/JB.183.6.2148-2150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang W., Allen S., Roberts C.J., Soultanas P. The Bacillus subtilis primosomal protein DnaD untwists supercoiled DNA. J. Bacteriol. 2006;188:5487–5493. doi: 10.1128/JB.00339-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang W., Machón C., Orta A., Phillips N., Roberts C.J., Allen S., Soultanas P. Single-molecule atomic force spectroscopy reveals that DnaD forms scaffolds and enhances duplex melting. J. Mol. Biol. 2008;377:706–714. doi: 10.1016/j.jmb.2008.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schneider S., Zhang W., Soultanas P., Paoli M. Structure of the N-Terminal Oligomerization Domain of DnaD Reveals a Unique Tetramerization Motif and Provides Insights into Scaffold Formation. J. Mol. Biol. 2008;376:1237–1250. doi: 10.1016/j.jmb.2007.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carneiro M.J.V.M., Zhang W., Ioannou C., Scott D.J., Allen S., Roberts C.J., Soultanas P. The DNA-remodelling activity of DnaD is the sum of oligomerization and DNA-binding activities on separate domains. Mol. Microbiol. 2006;60:917–924. doi: 10.1111/j.1365-2958.2006.05152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bruand C., Velten M., McGovern S., Marsin S., Sérèna C., Dusko Ehrlich S., Polard P. Functional interplay between the Bacillus subtilis DnaD and DnaB proteins essential for initiation and re-initiation of DNA replication. Mol. Microbiol. 2005;55:1138–1150. doi: 10.1111/j.1365-2958.2004.04451.x. [DOI] [PubMed] [Google Scholar]
- 104.Collier C., Machón C., Briggs G.S., Smits W.K., Soultanas P. Untwisting of the DNA helix stimulates the endonuclease activity of Bacillus subtilis Nth at AP sites. Nucleic Acids Res. 2012;40:739–750. doi: 10.1093/nar/gkr785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rannou O., Le Chatelier E., Larson M.A., Nouri H., Dalmais B., Laughton C., Jannière L., Soultanas P. Functional interplay of DnaE polymerase, DnaG primase and DnaC helicase within a ternary complex, and primase to polymerase hand-off during lagging strand DNA replication in Bacillus subtilis. Nucleic Acids Res. 2013;41:5303–5320. doi: 10.1093/nar/gkt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rokop M.E., Auchtung J.M., Grossman A.D. Control of DNA replication initiation by recruitment of an essential initiation protein to the membrane of Bacillus subtilis. Mol. Microbiol. 2004;52:1757–1767. doi: 10.1111/j.1365-2958.2004.04091.x. [DOI] [PubMed] [Google Scholar]
- 107.Grainger W.H., Machón C., Scott D.J., Soultanas P. DnaB proteolysis in vivo regulates oligomerization and its localization at oriC in Bacillus subtilis. Nucleic Acids Res. 2010;38:2851–2864. doi: 10.1093/nar/gkp1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hayashi M., Ogura Y., Harry E.J., Ogasawara N., Moriya S. Bacillus subtilis YabA is involved in determining the timing and synchrony of replication initiation. FEMS Microbiol. Lett. 2005;247:73–79. doi: 10.1016/j.femsle.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 109.Noirot-Gros M.-F., Dervyn E., Wu L.J., Mervelet P., Errington J., Ehrlich S.D., Noirot P. An expanded view of bacterial DNA replication. Proc. Natl. Acad. Sci. USA. 2002;99:8342–8347. doi: 10.1073/pnas.122040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Noirot-Gros M.-F., Velten M., Yoshimura M., McGovern S., Morimoto T., Ehrlich S.D., Ogasawara N., Polard P., Noirot P. Functional dissection of YabA, a negative regulator of DNA replication initiation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 2006;103:2368–2373. doi: 10.1073/pnas.0506914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Felicori L., Jameson K.H., Roblin P., Fogg M.J., Cherrier V., Bazin A., Garcia-garcia T., Ventroux M., Noirot P., Wilkinson A.J., et al. Tetramerization and interdomain flexibility of the replication initiation controller YabA enables simultaneous binding to multiple partners. Nucleic Acids Res. 2016;44:449–463. doi: 10.1093/nar/gkv1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scholefield G., Murray H. YabA and DnaD inhibit helix assembly of the DNA replication initiation protein DnaA. Mol. Microbiol. 2013;90:147–159. doi: 10.1111/mmi.12353. [DOI] [PubMed] [Google Scholar]
- 113.Cho E., Ogasawara N., Ishikawa S. The functional analysis of YabA, which interacts with DnaA and regulates initiation of chromosome replication in Bacillus subtils. Genes Genet. Syst. 2008;83:111–125. doi: 10.1266/ggs.83.111. [DOI] [PubMed] [Google Scholar]
- 114.Murray H., Errington J. Dynamic control of the DNA replication initiation protein DnaA by Soj/ParA. Cell. 2008;135:74–84. doi: 10.1016/j.cell.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 115.Scholefield G., Whiting R., Errington J., Murray H. Spo0J regulates the oligomeric state of Soj to trigger its switch from an activator to an inhibitor of DNA replication initiation. Mol. Microbiol. 2011;79:1089–1100. doi: 10.1111/j.1365-2958.2010.07507.x. [DOI] [PubMed] [Google Scholar]
- 116.Leonard T.A., Butler P.J., Löwe J. Bacterial chromosome segregation: Structure and DNA binding of the Soj dimer—A conserved biological switch. EMBO J. 2005;24:270–282. doi: 10.1038/sj.emboj.7600530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ringgaard S., van Zon J., Howard M., Gerdes K. Movement and equipositioning of plasmids by ParA filament disassembly. Proc. Natl. Acad. Sci. USA. 2009;106:19369–19374. doi: 10.1073/pnas.0908347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gerdes K., Howard M., Szardenings F. Pushing and pulling in prokaryotic DNA segregation. Cell. 2010;141:927–942. doi: 10.1016/j.cell.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 119.Lee P.S., Grossman A.D. The chromosome partitioning proteins Soj (ParA) and Spo0J (ParB) contribute to accurate chromosome partitioning, separation of replicated sister origins, and regulation of replication initiation in Bacillus subtilis. Mol. Microbiol. 2006;60:853–869. doi: 10.1111/j.1365-2958.2006.05140.x. [DOI] [PubMed] [Google Scholar]
- 120.Sullivan N.L., Marquis K.A., Rudner D.Z. Recruitment of SMC by ParB-parS Organizes the Origin Region and Promotes Efficient Chromosome Segregation. Cell. 2009;137:697–707. doi: 10.1016/j.cell.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gruber S., Errington J. Recruitment of Condensin to Replication Origin Regions by ParB/SpoOJ Promotes Chromosome Segregation in B. subtilis. Cell. 2009;137:685–696. doi: 10.1016/j.cell.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 122.Bonilla C.Y., Grossman A.D. The primosomal protein DnaD inhibits cooperative DNA binding by the replication initiator DnaA in Bacillus subtilis. J. Bacteriol. 2012;194:5110–5117. doi: 10.1128/JB.00958-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Okumura H., Yoshimura M., Ueki M., Oshima T., Ogasawara N., Ishikawa S. Regulation of chromosomal replication initiation by oriC-proximal DnaA-box clusters in Bacillus subtilis. Nucleic Acids Res. 2012;40:220–234. doi: 10.1093/nar/gkr716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Errington J. Regulation of endospore formation in Bacillus subtilis. Nat. Rev. Microbiol. 2003;1:117–126. doi: 10.1038/nrmicro750. [DOI] [PubMed] [Google Scholar]
- 125.Higgins D., Dworkin J. Recent progress in Bacillus subtilis sporulation. FEMS Microbiol. Rev. 2012;36:131–148. doi: 10.1111/j.1574-6976.2011.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kay D., Warren S.C. Sporulation in Bacillus subtilis. Morphological changes. Biochem. J. 1968;109:819–824. doi: 10.1042/bj1090819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiang M., Shao W., Perego M., Hoch J.A. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol. 2000;38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- 128.Molle V., Fujita M., Jensen S.T., Eichenberger P., González-Pastor J.E., Liu J.S., Losick R. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 2003;50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 129.Veening J.-W., Murray H., Errington J. A mechanism for cell cycle regulation of sporulation initiation in Bacillus subtilis. Genes Dev. 2009;23:1959–1970. doi: 10.1101/gad.528209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mandelstam J., Higgs S.A. Induction of sporulation during synchronized chromosome replication in Bacillus subtilis. J. Bacteriol. 1974;120:38–42. doi: 10.1128/jb.120.1.38-42.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dunn B.G., Jeffs P., Ma N.H. The Relationship Between DNA Replication and the Induction of Sporulation in Bacillus subtilis. Microbiology. 1978;108:189–195. doi: 10.1099/00221287-108-2-189. [DOI] [Google Scholar]
- 132.Narula J., Kuchina A., Lee D.D., Fujita M., Süel G.M., Igoshin O.A. Chromosomal Arrangement of Phosphorelay Genes Couples Sporulation and DNA Replication. Cell. 2015;162:328–337. doi: 10.1016/j.cell.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fujita M., González-Pastor J.E., Gonza E., Losick R. High- and Low-Threshold Genes in the Spo0A Regulon of Bacillus subtilis. J. Bacteriol. 2005;187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fujita M., Losick R. Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A. Genes Dev. 2005;19:2236–2244. doi: 10.1101/gad.1335705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burkholder W.F., Kurtser I., Grossman A.D. Replication initiation proteins regulate a developmental checkpoint in Bacillus subtilis. Cell. 2001;104:269–279. doi: 10.1016/S0092-8674(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 136.Bick M.J., Lamour V., Rajashankar K.R., Gordiyenko Y., Robinson C.V., Darst S.A. How to Switch Off a Histidine Kinase: Crystal Structure of Geobacillus stearothermophilus KinB with the inhibitor Sda. J. Mol. Biol. 2009;386:163–177. doi: 10.1016/j.jmb.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Whitten A.E., Jacques D.A., Hammouda B., Hanley T., King G.F., Guss J.M., Trewhella J., Langley D.B. The Structure of the KinA-Sda Complex Suggests an Allosteric Mechanism of Histidine Kinase Inhibition. J. Mol. Biol. 2007;368:407–420. doi: 10.1016/j.jmb.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 138.Ruvolo M.V., Mach K.E., Burkholder W.F. Proteolysis of the replication checkpoint protein Sda is necessary for the efficient initiation of sporulation after transient replication stress in Bacillus subtilis. Mol. Microbiol. 2006;60:1490–1508. doi: 10.1111/j.1365-2958.2006.05167.x. [DOI] [PubMed] [Google Scholar]
- 139.Levine J.H., Fontes M.E., Dworkin J., Elowitz M.B. Pulsed feedback defers cellular differentiation. PLoS Biol. 2012;10:e1001252. doi: 10.1371/journal.pbio.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chapman J.W., Piggot P.J. Analysis of the inhibition of sporulation of Bacillus subtilis caused by increasing the number of copies of the spo0F gene. J. Gen. Microbiol. 1987;133:2079–2088. doi: 10.1099/00221287-133-8-2079. [DOI] [PubMed] [Google Scholar]
- 141.Grimshaw C.E., Huang S., Hanstein C.G., Strauch M.A., Burbulys D., Wang L., Hoch J.A., Whiteley J.M. Synergistic kinetic interactions between components of the phosphorelay controlling sporulation in Bacillus subtilis. Biochemistry. 1998;37:1365–1375. doi: 10.1021/bi971917m. [DOI] [PubMed] [Google Scholar]
- 142.Rahn-Lee L., Gorbatyuk B., Skovgaard O., Losick R. The conserved sporulation protein YneE inhibits DNA replication in Bacillus subtilis. J. Bacteriol. 2009;191:3736–3739. doi: 10.1128/JB.00216-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wagner J.K., Marquis K.A., Rudner D.Z. SirA enforces diploidy by inhibiting the replication initiator DnaA during spore formation in Bacillus subtilis. Mol. Microbiol. 2009;73:963–974. doi: 10.1111/j.1365-2958.2009.06825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jameson K.H., Rostami N., Fogg M.J., Turkenburg J.P., Grahl A., Murray H., Wilkinson A.J. Structure and interactions of the Bacillus subtilis sporulation inhibitor of DNA replication, SirA, with domain I of DnaA. Mol. Microbiol. 2014;93:975–991. doi: 10.1111/mmi.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Duan Y., Huey J.D., Herman J.K. The DnaA inhibitor SirA acts in the same pathway as Soj (ParA) to facilitate oriC segregation during Bacillus subtilis sporulation. Mol. Microbiol. 2016;102:530–544. doi: 10.1111/mmi.13477. [DOI] [PubMed] [Google Scholar]
- 146.Boonstra M., de Jong I.G., Scholefield G., Murray H., Kuipers O.P., Veening J.-W.W. Spo0A regulates chromosome copy number during sporulation by directly binding to the origin of replication in Bacillus subtilis. Mol. Microbiol. 2013;87:925–938. doi: 10.1111/mmi.12141. [DOI] [PubMed] [Google Scholar]
- 147.Castilla-Llorente V., Muñoz-Espín D., Villar L., Salas M., Meijer W.J.J. Spo0A, the key transcriptional regulator for entrance into sporulation, is an inhibitor of DNA replication. EMBO J. 2006;25:3890–3899. doi: 10.1038/sj.emboj.7601266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Camara J.E., Breier A.M., Brendler T., Austin S., Cozzarelli N.R., Crooke E. Hda inactivation of DnaA is the predominant mechanism preventing hyperinitiation of Escherichia coli DNA replication. EMBO Rep. 2005;6:736–741. doi: 10.1038/sj.embor.7400467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Katayama T., Kubota T., Kurokawa K., Crooke E., Sekimizu K. The initiator function of DnaA protein is negatively regulated by the sliding clamp of the E. coli chromosomal replicase. Cell. 1998;94:61–71. doi: 10.1016/S0092-8674(00)81222-2. [DOI] [PubMed] [Google Scholar]
- 150.Kato J., Katayama T. Hda, a novel DnaA-related protein, regulates the replication cycle in Escherichia coli. EMBO J. 2001;20:4253–4262. doi: 10.1093/emboj/20.15.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Takata M., Kubota T., Matsuda Y., Katayama T. Molecular mechanism of DNA replication-coupled inactivation of the initiator protein in Escherichia coli: Interaction of DnaA with the sliding clamp-loaded DNA and the sliding clamp-Hda complex. Genes Cells. 2004;9:509–522. doi: 10.1111/j.1356-9597.2004.00741.x. [DOI] [PubMed] [Google Scholar]
- 152.Kurz M., Dalrymple B., Wijffels G., Kongsuwan K. Interaction of the Sliding Clamp Beta-Subunit and Hda, a DnaA-Related Protein. J. Bacteriol. 2004;186:3508–3515. doi: 10.1128/JB.186.11.3508-3515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Su’etsugu M., Shimuta T.-R.R., Ishida T., Kawakami H., Katayama T. Protein associations in DnaA-ATP hydrolysis mediated by the Hda-replicase clamp complex. J. Biol. Chem. 2005;280:6528–6536. doi: 10.1074/jbc.M412060200. [DOI] [PubMed] [Google Scholar]
- 154.Fujimitsu K., Su’etsugu M., Yamaguchi Y., Mazda K., Fu N., Kawakami H., Katayama T. Modes of overinitiation, dnaA gene expression, and inhibition of cell division in a novel cold-sensitive hda mutant of Escherichia coli. J. Bacteriol. 2008;190:5368–5381. doi: 10.1128/JB.00044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Su’etsugu M., Nakamura K., Keyamura K., Kudo Y., Katayama T. Hda Monomerization by ADP Binding Promotes Replicase Clamp-mediated DnaA-ATP Hydrolysis. J. Biol. Chem. 2008;283:36118–36131. doi: 10.1074/jbc.M803158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu Q., McMullan D., Abdubek P., Astakhova T., Carlton D., Chen C., Chiu H.-J., Clayton T., Das D., Deller M.C., et al. A structural basis for the regulatory inactivation of DnaA. J. Mol. Biol. 2009;385:368–380. doi: 10.1016/j.jmb.2008.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakamura K., Katayama T. Novel essential residues of Hda for interaction with DnaA in the regulatory inactivation of DnaA: Unique roles for Hda AAA + Box VI and VII motifs. Mol. Microbiol. 2010;76:302–317. doi: 10.1111/j.1365-2958.2010.07074.x. [DOI] [PubMed] [Google Scholar]
- 158.Keyamura K., Katayama T. DnaA Protein DNA-binding Domain Binds to Hda Protein to Promote Inter-AAA+ Domain Interaction Involved in Regulatory Inactivation of DnaA. J. Biol. Chem. 2011;286:29336–29346. doi: 10.1074/jbc.M111.233403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ryan V.T., Grimwade J.E., Camara J.E., Crooke E., Leonard A.C. Escherichia coli prereplication complex assembly is regulated by dynamic interplay among Fis, IHF and DnaA. Mol. Microbiol. 2004;51:1347–1359. doi: 10.1046/j.1365-2958.2003.03906.x. [DOI] [PubMed] [Google Scholar]
- 160.Kasho K., Fujimitsu K., Matoba T., Oshima T., Katayama T. Timely binding of IHF and Fis to DARS2 regulates ATP-DnaA production and replication initiation. Nucleic Acids Res. 2014;42:13134–13149. doi: 10.1093/nar/gku1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kasho K., Katayama T. DnaA binding locus datA promotes DnaA-ATP hydrolysis to enable cell cycle-coordinated replication initiation. Proc. Natl. Acad. Sci. USA. 2012;110:936–941. doi: 10.1073/pnas.1212070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Filutowicz M., Ross W., Wild J., Gourse R.L. Involvement of Fis protein in replication of the Escherichia coli chromosome. J. Bacteriol. 1992;174:398–407. doi: 10.1128/jb.174.2.398-407.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pratt T.S., Steiner T., Feldman L.S., Walker K.A., Osuna R. Deletion analysis of the fis promoter region in Escherichia coli: Antagonistic effects of integration host factor and Fis. J. Bacteriol. 1997;179:6367–6377. doi: 10.1128/jb.179.20.6367-6377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Flåtten I., Skarstad K. The Fis protein has a stimulating role in initiation of replication in Escherichia coli in vivo. PLoS ONE. 2013;8:1–9. doi: 10.1371/journal.pone.0083562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cassler M.R., Grimwade J.E., Leonard A.C. Cell cycle-specific changes in nucleoprotein complexes at a chromosomal replication origin. EMBO J. 1995;14:5833–5841. doi: 10.1002/j.1460-2075.1995.tb00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grimwade J.E., Ryan V.T., Leonard A.C. IHF redistributes bound initiator protein, DnaA, on supercoiled oriC of Escherichia coli. Mol. Microbiol. 2000;35:835–844. doi: 10.1046/j.1365-2958.2000.01755.x. [DOI] [PubMed] [Google Scholar]
- 167.Kitagawa R., Mitsuki H., Okazaki T., Ogawa T. A novel DnaA protein-binding site at 94.7 min on the Escherichia coli chromosome. Mol. Microbiol. 1996;19:1137–1147. doi: 10.1046/j.1365-2958.1996.453983.x. [DOI] [PubMed] [Google Scholar]
- 168.Kitagawa R., Ozaki T., Moriya S., Ogawa T. Negative control of replication initiation by a novel chromosomal locus exhibiting exceptional affinity for Escherichia coli DnaA protein. Genes Dev. 1998;12:3032–3043. doi: 10.1101/gad.12.19.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ogawa T., Yamada Y., Kuroda T., Kishi T., Moriya S. The datA locus predominantly contributes to the initiator titration mechanism in the control of replication initiation in Escherichia coli. Mol. Microbiol. 2002;44:1367–1375. doi: 10.1046/j.1365-2958.2002.02969.x. [DOI] [PubMed] [Google Scholar]
- 170.Nozaki S., Yamada Y., Ogawa T. Initiator titration complex formed at datA with the aid of IHF regulates replication timing in Escherichia coli. Genes Cells. 2009;14:329–341. doi: 10.1111/j.1365-2443.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 171.Fujimitsu K., Senriuchi T., Katayama T. Specific genomic sequences of E. coli promote replicational initiation by directly reactivating ADP-DnaA. Genes Dev. 2009;23:1221–1233. doi: 10.1101/gad.1775809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Frimodt-Møller J., Charbon G., Krogfelt K.A., Løbner-Olesen A. DNA Replication Control Is Linked to Genomic Positioning of Control Regions in Escherichia coli. PLoS Genet. 2016;12:1–27. doi: 10.1371/journal.pgen.1006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Inoue Y., Tanaka H., Kasho K., Fujimitsu K., Oshima T., Katayama T. Chromosomal location of the DnaA-reactivating sequence DARS2 is important to regulate timely initiation of DNA replication in Escherichia coli. Genes Cells. 2016;21:1015–1023. doi: 10.1111/gtc.12395. [DOI] [PubMed] [Google Scholar]
- 174.Slater S., Wold S., Lu M., Boye E., Skarstad K., Kleckner N. E. coli SeqA protein binds oriC in two different methyl-modulated reactions appropriate to its roles in DNA replication initiation and origin sequestration. Cell. 1995;82:927–936. doi: 10.1016/0092-8674(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 175.Lu M., Campbell J.L., Boye E., Kleckner N. SeqA: A negative modulator of replication initiation in E. coli. Cell. 1994;77:413–426. doi: 10.1016/0092-8674(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 176.Von Freiesleben U., Rasmussen K.V., Schaechter M. SeqA limits DnaA activity in replication from oriC in Escherichia coli. Mol. Microbiol. 1994;14:763–772. doi: 10.1111/j.1365-2958.1994.tb01313.x. [DOI] [PubMed] [Google Scholar]
- 177.Campbell J.L., Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell. 1990;62:967–979. doi: 10.1016/0092-8674(90)90271-F. [DOI] [PubMed] [Google Scholar]
- 178.Nievera C., Torgue J.J.-C., Grimwade J.E., Leonard A.C. SeqA Blocking of DnaA-oriC Interactions Ensures Staged Assembly of the E. coli Pre-RC. Mol. Cell. 2006;24:581–592. doi: 10.1016/j.molcel.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Han J.S., Kang S., Lee H., Kim H.K., Hwang D.S. Sequential binding of SeqA to paired hemi-methylated GATC sequences mediates formation of higher order complexes. J. Biol. Chem. 2003;278:34983–34989. doi: 10.1074/jbc.M304923200. [DOI] [PubMed] [Google Scholar]
- 180.Kang S., Lee H., Han J.S., Hwang D.S. Interaction of SeqA and Dam methylase on the hemimethylated origin of Escherichia coli chromosomal DNA replication. J. Biol. Chem. 1999;274:11463–11468. doi: 10.1074/jbc.274.17.11463. [DOI] [PubMed] [Google Scholar]
- 181.Waldminghaus T., Skarstad K. The Escherichia coli SeqA protein. Plasmid. 2009;61:141–150. doi: 10.1016/j.plasmid.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 182.Helgesen E., Fossum-Raunehaug S., Saetre F., Schink K.O., Skarstad K. Dynamic Escherichia coli SeqA complexes organize the newly replicated DNA at a considerable distance from the replisome. Nucleic Acids Res. 2015;43:2730–2743. doi: 10.1093/nar/gkv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zawilak-Pawlik A., Donczew R., Szafrański S., Mackiewicz P., Terradot L., Zakrzewska-Czerwińska J. DiaA/HobA and DnaA: A pair of proteins co-evolved to cooperate during bacterial orisome assembly. J. Mol. Biol. 2011;408:238–2351. doi: 10.1016/j.jmb.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 184.Keyamura K., Fujikawa N., Ishida T., Ozaki S., Su M., Fujimitsu K., Kagawa W., Yokoyama S., Kurumizaka H. The interaction of DiaA and DnaA regulates the replication cycle in E. coli by directly promoting ATP—DnaA-specific initiation complexes. Genes Dev. 2007;21:2083–2099. doi: 10.1101/gad.1561207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Natrajan G., Hall D.R., Thompson A.C., Gutsche I., Terradot L. Structural similarity between the DnaA-binding proteins HobA (HP1230) from Helicobacter pylori and DiaA from Escherichia coli. Mol. Microbiol. 2007;65:995–1005. doi: 10.1111/j.1365-2958.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- 186.Terradot L., Zawilak-Pawlik A. Structural insight into Helicobacter pylori DNA replication initiation. Gut Microbes. 2010;1:330–334. doi: 10.4161/gmic.1.5.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang Q., Zhou A., Li S.S., Ni J., Tao J., Lu J., Wan B., Li S.S., Zhang J., Zhao S., et al. Reversible lysine acetylation is involved in DNA replication initiation by regulating activities of initiator DnaA in Escherichia coli. Sci. Rep. 2016;6:30837. doi: 10.1038/srep30837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Leonard A.C., Grimwade J.E. Regulating DnaA complex assembly: It is time to fill the gaps. Curr. Opin. Microbiol. 2010;13:766–772. doi: 10.1016/j.mib.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]