Abstract
Verticillium dahliae invades the roots of host plants and causes vascular wilt, which seriously diminishes the yield of cotton and other important crops. The protein AAC (ADP, ATP carrier) is responsible for transferring ATP from the mitochondria into the cytoplasm. When V. dahliae protoplasts were transformed with short interfering RNAs (siRNAs) targeting the VdAAC gene, fungal growth and sporulation were significantly inhibited. To further confirm a role for VdAAC in fungal development, we generated knockout mutants (ΔVdACC). Compared with wild-type V. dahliae (Vd wt), ΔVdAAC was impaired in germination and virulence; these impairments were rescued in the complementary strains (ΔVdAAC-C). Moreover, when an RNAi construct of VdAAC under the control of the 35S promoter was used to transform Nicotiana benthamiana, the expression of VdAAC was downregulated in the transgenic seedlings, and they had elevated resistance against V. dahliae. The results of this study suggest that VdAAC contributes to fungal development, virulence and is a promising candidate gene to control V. dahliae. In addition, RNAi is a highly efficient way to silence fungal genes and provides a novel strategy to improve disease resistance in plants.
Keywords: Verticillium dahliae, VdAAC, RNAi, growth, virulence
1. Introduction
Verticillium dahliae is one of the most destructive soil-borne fungi, infecting many important economic crops, fruit trees and ornamental flowers [1,2]. This fungus can cause typical disease symptoms, including stunted growth, necrosis, wilt and defoliation, which severely decrease the yield and quality of crops [3]. Each year, Verticillium wilt is reported to cause extensive economic losses to the crop industry [4]. The fungus can survive in soil for many years and infect the roots of its hosts. Its mycelium then abundantly colonizes the vascular bundle to block the transportation of nutrients [5]. Once the fungus is established in the host, Verticillium wilt is an intractable disease because of the intricate pathogenic mechanism of V. dahliae [6]. Currently, no fungicide is available to cure the disease caused by this fungus [7]. Previous studies on V. dahliae have thus focused on identifying genes that are crucial for fungal development and virulence [8,9,10], inestimable knowledge for crop breeding programs.
RNA interference (RNAi) is an effective tool to investigate gene function and elevate plant resistance against a fungus [11,12,13]. In V. longisporum, the expression of Vlaro2 was reduced via RNAi, resulting in a bradytrophic mutant [14]. In vitro cultures of Fusarium graminearum, the introduction of double-stranded (ds)RNA that targeted cytochrome P450 lanosterol C14α-demethylase-encoding genes inhibited fungal growth. Similarly, expressing the same region of dsRNA into susceptible Arabidopsis thaliana and Hordeum vulgare conferred high resistance to fungal infection [15]. Transgenic banana plants with siRNAs targeted against velvet and Fusarium transcription factor 1 were protected against Fusarium oxysporum f. sp. cubense (Foc) [16], as were transgenic cotton plants against V. dahliae when the fungal VdH1 gene was silenced [17].
Genes for essential cellular components may prove to be likely effective targets. For example, mitochondrial carriers are a series of proteins that transport nucleotides, amino acids, fatty acids, and so on across the inner mitochondrial membrane of eukaryotes [18]. Of these carriers, the highly conserved AAC is the most abundant protein [19,20]. AAC is essential for maintaining fluxes in energy and mediating the exchange of ADP and ATP between the mitochondria and cytoplasm [21]. AAC consists of six transmembrane helices embedded in the inner mitochondrial membrane with its N- and C-terminals exposed to the cytosolic side [22]. The C-terminal structure of yeast AAC is predicted to be involved in regulating the accessibility of the transmembrane core to water [23]. Silencing of the AAC gene of Blumeria graminis by biolistically bombarding barley cells with RNAi constructs led to the formation of fewer haustoria in barley cells [24]. In Saccharomyces cerevisiae, AAC might transmit a signal and facilitate permeabilization of the outer mitochondrial membrane to accelerate mitochondrial degradation followed by cytochrome C release during acetic acid-induced apoptosis [25]. Thylakoid ADP/ATP carrier (TAAC), apart from regulating ADP and ATP balance, has an additional role in transporting 3′-Phosphoadenosine 5′-phosphosulfate as the high-energy sulfate donor through the plastid envelope in Arabidopsis [26]. AAC also increases mitochondrial proton conductance for adapting to cold water stress in king penguins [27]. A decrease in the expression of Trypanosoma brucei AAC resulted in a reduced level of cytosolic ATP and mitochondrial oxygen consumption, severe growth defects and elevation in the amount of reactive oxygen species [28].
Although the functions of the AAC gene in development, resistance and signal transduction pathways have been explored in other species, its role in the development and virulence of V. dahliae has not yet been reported. In the present study, we used siRNA-induced silencing of the VdAAC gene in V. dahliae to establish the relationship between VdAAC and fungal development. Deletion of VdAAC resulted in reduced colony growth and sporulation. Virulence was significantly decreased in the ΔVdAAC mutants compared with the wild type (Vd wt) and complemented strains (ΔVdAAC-C). Confocal microscopic observations revealed that conidial germination of ΔVdAAC was significantly impaired. Moreover, transgenic N. benthamiana expressing dsRNA against VdAAC showed strong resistance against V. dahliae. Our results indicate that VdAAC contributes to fungal germination, development and sporulation, which are requisite for the fungus to invade the plants and induce full virulence in the host. For potential exploitation of this gene to protect crops against V. dahliae, its biological function needs to be further elaborated.
2. Materials and Methods
2.1. Fungal Strains, Plants and Inoculation with V. dahliae
Strain V991, a highly toxic and defoliating wild-type pathogenic strain of V. dahliae, was kindly gifted by Prof. Guiliang Jian of the Institute of Plant Protection, Chinese Academy of Agricultural Sciences (CAAS). V. dahliae strain Vd-GFP that expresses GFP, is from our laboratory culture collection [29]. Single-conidium cultures of all V. dahliae strains were grown in complete medium broth (CM) at 25 °C. After 1 week, the conidia were harvested for inoculation.
Two-week-old seedlings of N. benthamiana were transplanted from Murashige-Skoog (MS) agar into disinfested soil and incubated in the greenhouse at 23 ± 2 °C, 75% ± 5% relative humidity, and a photoperiod of 16 h day/8 h night. After 1 month, seedlings with 6–8 leaves were inoculated by dipping the roots in a suspension of 106 conidia·mL−1 for 2 min.
2.2. Disease Index
Disease severity was evaluated using a five-grade scale based on a previous study with modifications [30]: grade 0, no wilt; grade 1, less than two leaves wilting; grade 2, three to five leaves wilting; grade 3, more than five leaves wilting or chlorotic; and grade 4, plant death or near death. Each respective experiment comprised 5 seedlings and was independently repeated three times for each assessment. Symptoms were recorded and the disease index (DI) calculated at 10 days post inoculation (dpi), 11 dpi and 12 dpi using the formula: DI = [Ʃ (number × grade)/(5 × 4)] × 100 [30,31].
2.3. Bioinformatics Analysis
The whole sequence of VdAAC was obtained from the Verticillium genomic database (www.broadinstitute.org). Amino acid sequences homologous to AAC in other species, identified with a Blastp search of the Protein Data Base (PDB), were used to construct a phylogenetic tree in MEGA software (version 6.06) (http://www.megasoftware.net/).
2.4. siRNA Design and Transformation of V. dahliae Protoplasts
The siRNAs targeting different regions of the VdAAC gene (siRNA-1, siRNA-2, siRNA-3 and siRNA-4) were designed using BLOCK-iT™ (Invitrogen, Carlsbad, CA, USA) RNAi Designer and synthesized by Oligobio, Beijing, China. The siRNAs sequences are given in Table 1, and the locations of these siRNAs in VdAAC are displayed in Figure S1A.
Table 1.
siRNA sequences designed against VdAAC.
| Name | Sense Sequence | Antisense Sequence |
|---|---|---|
| Control | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| siRNA-1 | UCAAGCUCCUCAUCCAGAATT | UUCUGGAUGAGGAGCUUGATT |
| siRNA-2 | GCAACACUGCCAACGUCAUTT | AUGACGUUGGCAGUGUUGCTT |
| siRNA-3 | GCUUUCCGUGACAAGUUCATT | UGAACUUGUCACGGAAAGCTT |
| siRNA-4 | GCAUGUACGACUCCAUCAATT | UUGAUGGAGUCGUACAUGCTT |
V. dahliae protoplasts isolated from fresh mycelia were transformed with the siRNAs as described in our previous study [29]. After 72 h in TB3 broth, the mycelia were collected to extract RNA with an RNA Extraction Kit (YPHBio, Tianjin, China). First strand cDNA was synthesized using a Reverse Transcription Kit (TransGen, Beijing, China) based on the manufacturer’s instructions. qRT-PCR was carried out with a 7500 Real Time PCR System (ABI, Foster City, CA, USA) [29]. Vdactin was used as a housekeeping gene [32]. The relative expression level of VdAAC was analyzed using the 2−ΔΔCt method. The standard curve met the experimental requirements (R2 > 0.99, E > 95%) [33]. Transformed protoplasts were also cultured for 2 weeks on the center of PDA (Potato Dextrosa Agar: potato infusion 200 g, dextrose 20 g and agar 20 g in 1 L H2O) plates to measure colony diameter and count the conidia produced to assess the effect of silencing on fungal growth and sporulation.
2.5. Plasmid Construction and Fungal Transformation
For creating a knockout-infused gene fragment, flanking regions (1 kbp upstream and downstream) of the VdAAC gene and a hygromycin resistance (HPT) expression cassette were amplified and fused via the overlapping sequences.
For GFP (Green Fluorescence Protein) disruption mutants (ΔVdAAC-GFP), the neomycin resistance (NeoR) cassette containing XbaI and BstEII restriction sites was cloned into the pCAMBIA1302 vector. After that, the GFP expression cassette was introduced into the plasmid via XbaI and KpnI restriction sites to generate pCAMBIA1302::Neo::GFP. Meanwhile, the VdAAC ORF was substituted for the GFP open reading frame (ORF) of the recombinant plasmid as pCAMBIA1302::Neo::VdAAC for complementary strains.
The respective constructs (knockout-infused fragment, pCAMBIA1302::Neo::GFP, pCAMBIA1302::Neo::VdAAC) were used to transform V. dahliae protoplasts [29]. Transformants were selected based on antibiotic resistance and confirmed by RT-PCR. The primers are listed in Table 2.
Table 2.
Primers and their sequences used in this study.
| Primers | Sequence |
|---|---|
| qRT-AAC | TTGCCGAGTGCTTCAAGCGTAC |
| GGCGTAGTCGAGGGAGTAGACG | |
| qRT-Vdactin | GGCTTCCTCAAGGTCGGCTATG |
| GCTGCATGTCATCCCACTTCTTC | |
| qRT-VdITS | CCGCCGGTCCATCAGTCTCTCTGTTTATAC |
| CGCCTGCGGGACTCCGATGCGAGCTGTAAC | |
| qRT-Nbactin | GGACCTTTATGGAAACATTGTGCTCAGT |
| CCAAGATAGAACCTCCAATCCAGACAC | |
| qRT-VA | GGGTATTCAGACCCTATTGGACG |
| CGAACTTCTTGTACTCAGCCTCC | |
| qRT-ATP6 | CTAGACCAATTTGAAATAAGA |
| AAAGATTCTTGGCTAATAGAT | |
| qRT-VdAC | TCTCCATCGTCTTCACCGACATCA |
| TCTGCACGGCGAAACACCACA | |
| qRT-VdATP-PRT | CGACGCCAACGTGCGGTCCTACAA |
| GCCCGAGAAGCTCGTGCCAAT | |
| HPT expression cassette | TTGAAGGAGCATTTTTGGGC |
| TTATCTTTGCGAACCCAGGG | |
| ΔAAC | CTTGGTGAAGGAGAGCGTTGAAAGT |
| GCCCAAAAATGCTCCTTCAATGACAAGTTCAAGGCCATGTTCGGC | |
| CCCTGGGTTCGCAAAGATAACTCCGTTGCTGGTATCGTTGTCTAC | |
| GGTTCCTCGTCGCTGTCAATGACC | |
| Neo expression cassette | aatTCTAGAGTTTGCGGGCTGTCTTGACG |
| ataGGTCACCTACCTGTGCATTCTGGGTAA | |
| GFP expression cassette | ggcTCTAGACTTTCGACACTGAAATACGTCG |
| ataGGTACCGCATCAGAGCAGATTGTACTGAGAG | |
| ΔAAC-C | aaaAGTACTATGTCCGTCGAGAAGCAG |
| aaaCTGCAGTTATTTGAAGGCCTTGCC | |
| Trans-AAC | GGGGACAAGTTTGTACAAAAAAGCAGGCTGTGCTTCAAGCGTAC |
| GGGGACCACTTTGTACAAGAAAGCTGGGTCCCTTGAAGAGAGAC | |
| Det-trans | CGTCATCCGTTACTTCCCTACCCA |
| AGACCGGCAATACCGTCAGAGGC | |
| Det-GFP | CGACGTAAACGGCCACAAGTT |
| TCTTTGCTCAGGGCGGACTGG | |
| Det-AAC | GCGCCAGTTCAACGGTCTTGTCG |
| TCACCAGAGGTCATCATCATGCGAC | |
| Det-Neo | GTTGTCACTGAAGCGGGAAGGG |
| GCGATACCGTAAAGCACGAGGAA | |
| Det-HPT | TTCGACAGCGTCTCCGACCTGA |
| AGATGTTGGCGACCTCGTATTGGG |
Restriction enzyme sites are indicated using bold and italic fonts.
2.6. Stress Treatments of V. dahliae Strains
For characterizing the development and morphology of the wild type and mutant strains of V. dahliae, 10 µL samples of 1 × 106 conidia·mL−1 of the respective strains were cultured on Czapek-Dox (3 g NaNO3, 1 g K2HPO4, 0.5 g MgSO4.7H2O, 0.5 g KCl, 0.01g FeSO4 and 30 g Sucrose in 1 L H2O) agar with either 0.5 M NaCl or sorbitol. Similarly, plates containing conidia of the respective strains were exposed to UV light for 10 s in a Gel doc system (Syngene, Cambridge, UK) to assess the impact of UV-stress on the survival of these conidia [34]. The plates were then incubated at 25 °C and the colony diameter on each plate was measured after 2 weeks. For estimating conidia production, 3 mL of sterilized water was added to each plate, which was then gently shaken to release the conidia [35]. The conidia were then counted using a light microscope (BX52, OLYMPUS, Tokyo, Japan).
2.7. Confocal Microscopy
Confocal microscopy was used to facilitate the observation of the infection process of both wild type and mutant strains. N. benthamiana seedlings were inoculated with Vd-GFP and ΔVdAAC-GFP strains respectively. At 7 dpi, roots of the infected plants were collected, washed with water for 3 times and then observed under confocal microscope (LSM 700, Carl Zeiss, Jena, Germany) [36].
2.8. Plasmid Construction and Plant Transformation
A pair of primers was designed based on the VdAAC ORF [37] (Table 2). The targeted fragment (648 bp) in VdAAC, shown in Figure S2A, was amplified with partial BP adaptors. The whole sequence was cloned using BP site primers and inserted into pDONR207 by a BP recombinant reaction (Invitrogen, Carlsbad, CA, USA). Then, the targeted fragment was cloned into the pK7GWIWG2(I) vector using an LR recombinant reaction (Invitrogen, Carlsbad, CA, USA). The recombinant plasmid was named pK7GWIWG2(I)-VdAAC (Figure S2B), confirmed by sequencing, and then used to transform Agrobacterium tumefaciens strain LBA4404 using electroporation [38].
Sterile leaves of N. benthamiana were immersed in A. tumefaciens strain LBA4404 containing the recombinant plasmid and transferred to MS agar. After 3 days, the leaves were cultured on MS agar containing 100 mg·L−1 kanamycin [39]. Seedlings were confirmed by PCR to be transgenic (Figure S2C). Two transgenic lines (Trans-1 and Trans-2), expressing dsRNA against VdAAC were selected for further analysis. The primer sequences for detection are listed in Table 2.
2.9. Analysis of Fungal Biomass
Colonization of V. dahliae in seedlings was quantified at 12 dpi by isolating DNA from the roots, stems (0–3 cm above the soil line) and leaves, respectively, using the Plant Genomic DNA Kit (TIANGEN, Beijing, China). Fungal biomass was quantified via qRT-PCR by amplifying ITS1 and ITS2 of rDNA (Z29511) of V. dahliae [35]. The N. benthamiana housekeeping gene (Nbactin) was used as an internal control [40]. The primers are listed in Table 2.
2.10. qRT-PCR Analysis of the Expression Level of Targeted Genes
The silencing effect of VdAAC in the infected seedlings was assessed by extracting RNA from roots at 12 dpi for qRT-PCR as described in Section 2.4. The housekeeping gene Vdactin was used as a control [32]. The relative expression of targeted gene was analyzed using the 2−∆∆Ct method. The standard curve met experimental requirements (R2 > 0.99, E > 95%) [33]. The primers are listed in Table 2.
2.11. Statistical Analysis
All experiments were independently repeated thrice, and data was analyzed for significant differences among the groups using Duncan’s multiple range test (p < 0.05) and SPSS Statistics 17.0 software (SPSS, Chicago, IL, USA).
3. Results
3.1. Bioinformatics Analysis of VdAAC
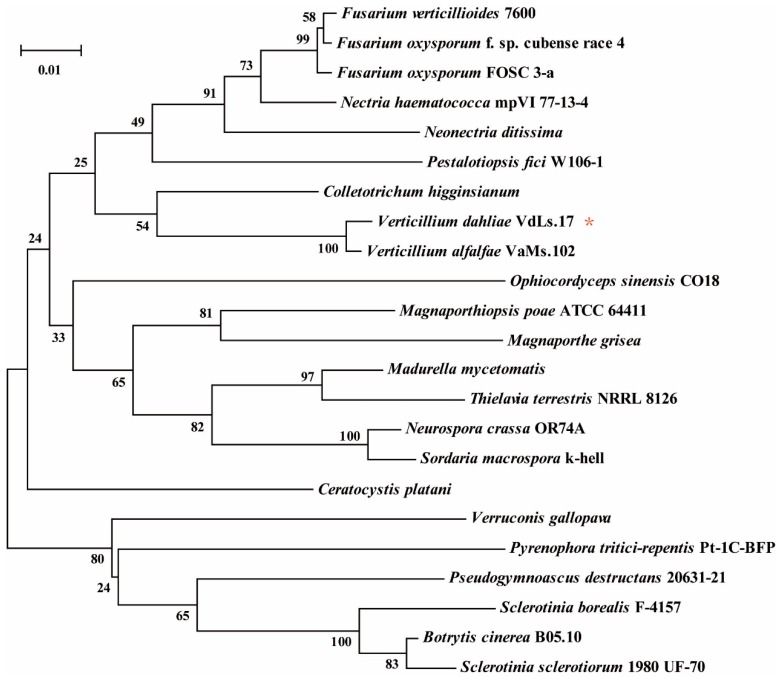
The ORF of VdAAC (VDAG_07535.1) contains 930 bp, which encodes a protein with 310 amino acids (GenBank NO.: XP_009654735.1). The neighbor-joining phylogenetic tree for the VdAAC sequences from V. dahliae and other fungi constructed using MEGA (bootstraps: 1000) demonstrated that the AAC sequences are relatively conserved among these fungal species (Figure 1).
Figure 1.
Phylogenetic analysis of AAC amino acid sequences from different fungal species. The phylogenetic tree was constructed by MEGA software (version 6.06; bootstraps: 1000). Fungal species and protein accession numbers: Verticillium dahliae VdLs.17 (XP_009654735.1); Nectria haematococca mpVI 77-13-4 (XP_003051617.1); Fusarium oxysporum FOSC 3-a (EWZ02370.1); Verticillium alfalfae VaMs.102 (XP_003004480.1); Fusarium verticillioides 7600 (EWG42987.1); Pestalotiopsis fici W106-1 (XP_007835574.1); Fusarium oxysporum f. sp. cubense race 4 (EMT68221.1); Magnaporthiopsis poae ATCC 64411 (KLU91586.1); Neonectria ditissima (KPM44251.1); Madurella mycetomatis (KOP45712.1); Colletotrichum higginsianum (CCF32866.1); Ceratocystis platani (KKF94862.1); Pseudogymnoascus destructans 20631-21 (XP_012739498.1); Botrytis cinerea B05.10 (XP_001559435.1); Sclerotinia sclerotiorum 1980 UF-70 (XP_001598713.1); Verruconis gallopava (KIW06207.1); Sclerotinia borealis F-4157 (ESZ96107.1); Neurospora crassa OR74A (XP_011393638.1); Sordaria macrospora k-hell (XP_003351160.1); Pyrenophora tritici-repentis Pt-1C-BFP (XP_001934086.1); Ophiocordyceps sinensis CO18 (EQK99145.1); Magnaporthe grisea (AAX07662.1); Thielavia terrestris NRRL 8126 (XP_003658188.1). * represents the query sequence.
3.2. Silencing of VdAAC Effectively Inhibited Fungal Growth and Sporulation
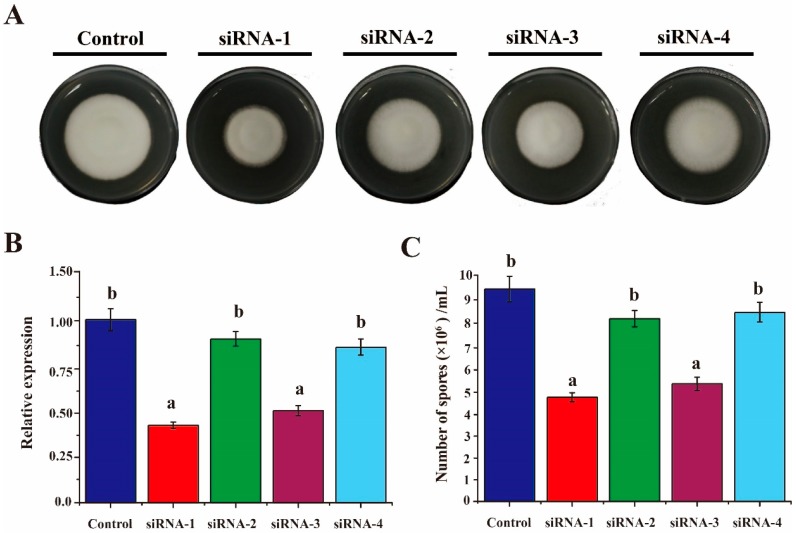
In our previous study [29], we showed that the siRNAs can enter V. dahliae protoplasts to silence the targeted genes. Thus, siRNAs designed against VdAAC were used to transform the protoplasts. After 2 weeks, the mean colony diameter of the siRNA-1 group (14.2 mm) and siRNA-3 (16 mm) were distinctly smaller than that of the siRNA-control group (24.5 mm) (Figure 2A and Figure S1B). To further confirm whether the silencing of VdAAC gene led to the reduced colony growth, qRT-PCR was carried out to determine the relative expression level of VdAAC in all the groups. The data was consistent with the colony diameter assessment (Figure 2B). Similarly, the siRNA-1 and siRNA-3 groups produced fewer conidia than the other groups did (Figure 2C). Taken together, these results demonstrate that inhibition of VdAAC expression impairs the fungal growth and sporulation.
Figure 2.
Assay for siRNA inhibition of the VdAAC gene. V. dahliae protoplasts were transformed with 10 μM siRNA-1, siRNA-2, siRNA-3, siRNA-4 or control, respectively. After regenerating for 18 h in TB3 broth, the protoplasts were cultured in the center of a PDA plate. (A) Colony morphology on PDA after 2 weeks; (B) Relative expression levels of VdAAC in different RNAi-treated groups. RNA was extracted from mycelia 72 h after transformation. First strand cDNA was synthesized, and qRT-PCR was carried out; (C) Number of conidia produced by the control and siRNA groups after 2 weeks. Error bars represent standard deviation (SD) calculated from means for three independent replicates. Significant differences (p < 0.05) among means for the different incubation times in Duncan’s multiple range test are indicated with different letters.
3.3. Generation of the VdAAC Mutant
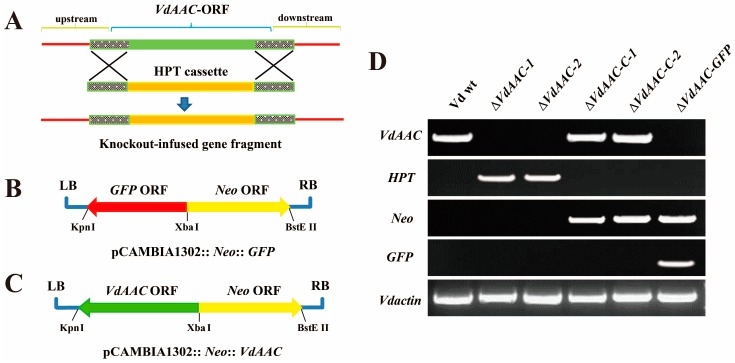
To further explore the function of VdAAC, we used the knockout-infused fragment to transform Vd wt protoplasts to generate the VdAAC deletion mutants (Figure 3A). pCAMBIA1302::Neo::GFP was used to transform the gene deletion strains to facilitate confocal microscopy while pCAMPIA1302::Neo::VdAAC for complementation assays (Figure 3B,C).
Figure 3.
Disruption of the VdAAC gene and confirmation of V. dahliae mutants. (A) Construction of the knockout-infused fragment for gene disruption. The fragment was obtained by fusing about 1 kb upstream and downstream of the VdAAC gene and hygromycin resistance (HPT) cassette; (B) GFP expression cassette (GFP) and neomycin resistance (Neo) cassette were introduced into pCAMBIA1302 to generate pCAMBIA1302::Neo::GFP; (C) GFP expression cassette (GFP) was repalced by VdAAC expression cassette (VdAAC) to produce pCAMBIA1302::Neo::VdAAC; (D) Confirmation of transformants. RNA was isolated from mycelia of mutants cultured in CM broth. The first strand cDNA was synthesized, and RT-PCR was carried out to confirm the transformants. Vdactin gene was used as a housekeeping gene.
Subsequently, transformants of ΔVdAAC (VdAAC deletion mutant), ΔVdAAC-C (VdAAC complementation mutant, obtained from transforming pCAMPIA1302::Neo::VdAAC into ΔVdAAC) and ΔVdAAC-GFP (VdAAC mutant transformed with GFP plasmid) strains were selected randomly and anlayzed by PCR (Figure 3D). Two gene deletion strains ΔVdAAC-1 and ΔVdAAC-2 and two complementary strains ΔVdAAC-C-1 (derived from the transformation of ΔVdAAC-1) and ΔVdAAC-C-2 (derived from the transformation of ΔVdAAC-2) were selected for further work. As expected, VdAAC expression was only detected in Vd wt, ΔVdAAC-C-1 and ΔVdAAC-C-2, and not in ΔVdAAC-1 and ΔVdAAC-2. Moreover, GFP expression was detected in ΔVdAAC-GFP. The transformants were then further analyzed for the role of VdAAC in the development and virulence of V. dahliae.
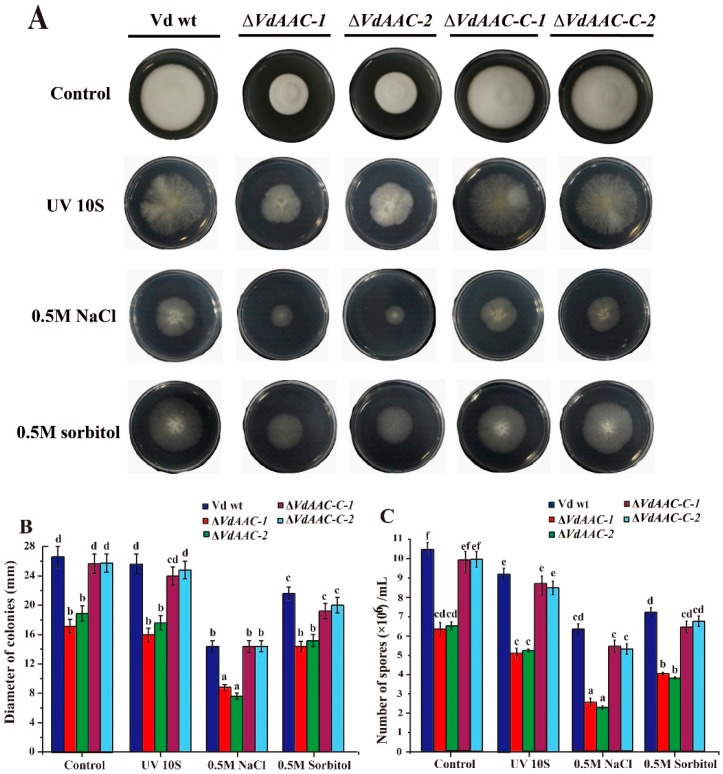
3.4. Stress Response Assay
The function of VdAAC in stress responses was analyzed by exposing conidia of Vd wt, ΔVdAAC, and ΔVdAAC-C strains to UV light, high NaCl or sorbitol. The phenotype of all the strains in the absence of stress was much better (Figure 4A). Exposure to each stress resulted in no significant reduction in the colony diameters and conidial number of ΔVdAAC when compared with the effect of the stress on Vd wt and ΔVdAAC-C (Figure 4A,B). Although the effect of NaCl and sorbitol stresses was significant on the growth of all the strains, however the ration of growth reduction for gene deletion mutants with wild type and complementary strains was similar to no stress conditions (Figure 4C). In brief, VdAAC might not have a significant contribution in stress tolerance.
Figure 4.
Colony morphology, diameter and conidia number of ΔVdAAC, ΔVdAAC-C and wild-type V. dahliae (Vd wt) strains exposed to stresses and without stress. Conidia from the respective strains were exposed to UV light and cultured in the center of Czapek-Dox agar plates. Conidia without UV light exposure were cultured on media supplemented with either NaCl or sorbitol. After 2 weeks, fungal traits were assessed: (A) colony morphology; (B) colony diameter; and (C) conidia number of mutants and Vd wt strain. Different letters (a–f) above the bars represent significant differences among the treatment groups (p < 0.05) as determined by the Duncan’s multiple range test.
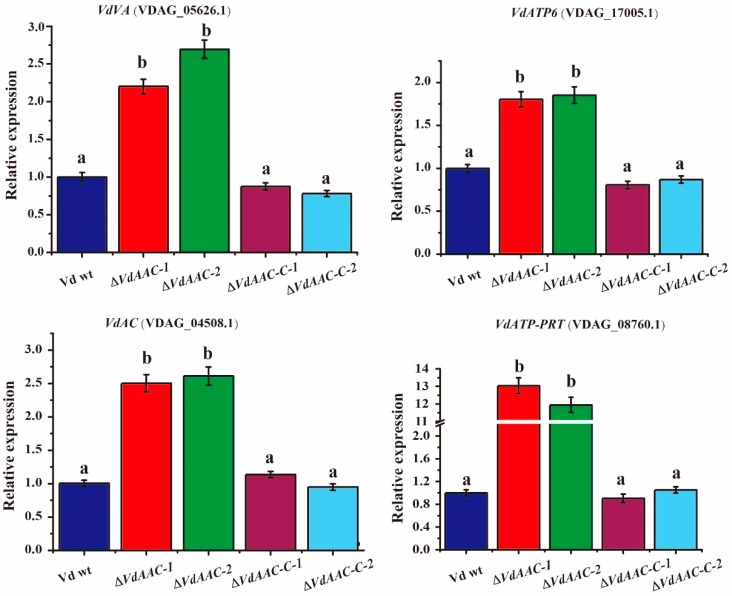
3.5. Expression of Genes Involved in Energy Metabolism in ΔVdAAC
To understand the regulation of target genes relevant to energy metabolism, we analyzed the expression of vacuolar ATPase (VDAG_05626.1, VdVA), a gene that is involved in generating electrochemical potential across the vacuolar membrane [41,42,43], ATP synthase F0 subunit 6 (VDAG_17005.1, VdATP6), responsible for phosphorylating ADP [44,45], adenylate cyclase (VDAG_04508.1, VdAC), required for the conversion of ATP to cAMP [46,47] and ATP phosphoribosyltransferase (VDAG_08760.1, VdATP-PRT), necessary for the formation of phosphoribosyl-ATP and inorganic pyrophosphate [48,49,50,51] in Vd wt, ΔVdAAC and ΔVdAAC-C. The expression of these genes in ΔVdAAC strains increased >2-fold as compared with Vd wt and ΔVdAAC-C (Figure 5). Collectively, these results suggest that the upregulation of these genes might be due to the disturbance in ADP/ATP levels.
Figure 5.
Relative expression of related genes involved in energy metabolism. Conidia from the respective strains were cultured in CM broth. After 4 days, the mycelia were collected for RNA extraction and the cDNA was synthesized. Expression patterns of four genes, vacuolar ATPase (VDAG_05626.1, VdVA), ATP synthase F0 subunit 6 (VDAG_17005.1, VdATP6), adenylate cyclase (VDAG_04508.1, VdAC), ATP phosphoribosyltransferase (VDAG_08760.1, VdATP-PRT), were determined by qRT-PCR. Vdactin gene was used as the reference gene for the expression analysis. Significant differences among the treatment groups (p < 0.05) are indicated by different letters (a, b) above the bars determined by the Duncan’s multiple range test.
3.6. VdAAC Is Involved in Fungal Virulence
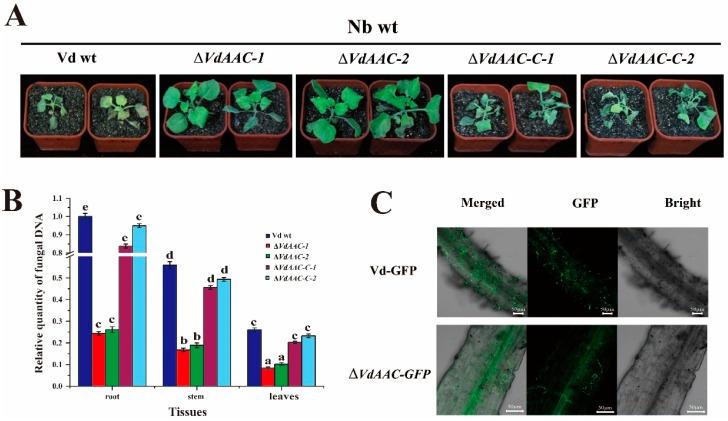
For evaluating the consequences of VdAAC deletion on fungal virulence, wild-type N. benthamiana (Nb wt) seedlings were inoculated with Vd wt, ΔVdAAC, and ΔVdAAC-C. At 12 dpi, seedlings inoculated with the Vd wt exhibited typical wilting symptoms and seemed nearly dead. In contrast, the disease index of seedlings inoculated with ΔVdAAC remained at a low level and was 70%–80% lower than that of the Vd wt group. The lower leaves of plants displayed a mild necrosis. The symptoms and disease index of seedlings inoculated with ΔVdAAC-C were similar to that of the Vd wt group (Figure 6A and Figure S3A). Fungal biomass in the various tissues of the plants inoculated with the different strains was then analyzed using qRT-PCR (Figure 6B). Fungal biomass of ΔVdAAC was significantly lower than that of the Vd wt and ΔVdAAC. These results were consistent with the phenotype and disease index data.
Figure 6.
Virulence analysis of ΔVdAAC, ΔVdAAC-C and wild-type V. dahliae (Vd wt) strains on the wild-type Nicotiana benthamiana (Nb wt). The Nb wt seedlings were inoculated with 106 conidia·mL−1. (A) Virulence phenotypes of Nb wt seedlings and (B) fungal biomass of different tissues of plants at 12 days after inoculation with the different strains. For the relative quantitative analysis, ITS1 and ITS2 of rDNA (Z29511) of V. dahliae were quantified relative to N. benthamiana housekeeping gene (Nbactin) for equilibration. (C) GFP fluorescence detection in roots of plants 7 days after inoculation with Vd-GFP or ΔVdAAC-GFP. Duncan’s multiple range test was applied to determine significant differences among the treatment groups (p < 0.05) indicated by different letters (a–e).
To investigate the VdAAC effect on fungal germination, 103 conidia of Vd-GFP and ΔVdAAC-GFP strains were added to PDA plates. After 48 h, the germination of ΔVdAAC-GFP conidia was nearly half that of Vd-GFP (Figure S3B). Furthermore, when the infection process of Vd-GFP and ΔVdAAC-GFP strains was examined microscopically (Figure 6C), at 7 dpi, many conidia of Vd-GFP had germinated, and hyphae were growing on the root surface. In contrast, fewer ΔVdAAC-GFP conidia had germinated compared with Vd-GFP. Meanwhile, the fungal biomass was lower on the root surface. All these results demonstrate that VdAAC contributes to fungal germination and growth, requisite for invasion and full virulence.
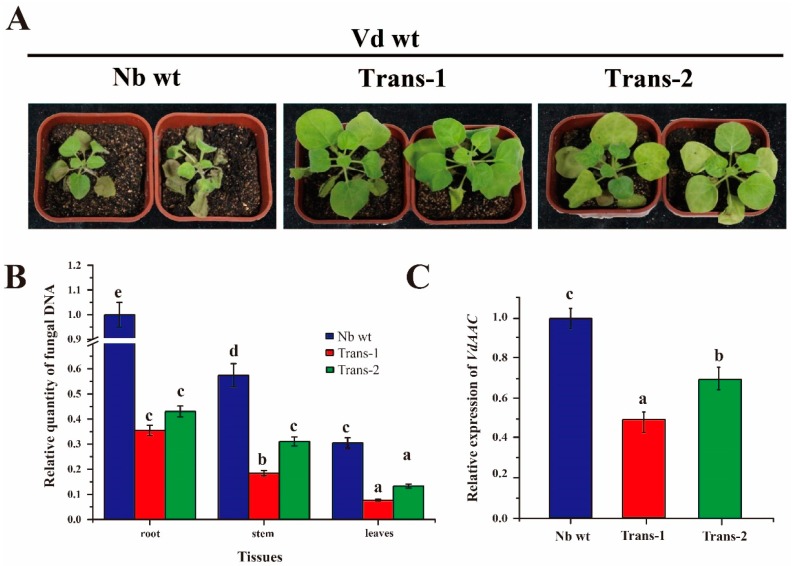
3.7. DsRNA of VdAAC Confers Resistance against Vd in Transgenic Lines
Transgenic seedlings (Trans-1 and Trans-2) were inoculated with fungal Vd wt conidia to validate whether dsVdAAC can confer resistance against V. dahliae (Figure 7A and Figure S2D). From 10 dpi, the Nb wt group displayed typical wilt symptoms, and the disease index was more than 80. At 12 dpi, the seedlings of the Nb wt group were nearly dead, and the disease index was approximately 100. In contrast, at 10 dpi, seedlings of the transgenic groups had weak symptoms. At 12 dpi, the disease index was 70% lower in the Trans-1 group and 36% lower in the Trans-2 group than in the Nb wt group.
Figure 7.
Assessment of the transgenic Nicotiana benthamiana for resistance against wild-type V. dahliae. One-month-old wild-type Nicotiana benthamiana (Nb wt) and transgenic lines (Trans-1 and -2) seedlings were inoculated with Vd wt and analyzed at 12 dpi. (A) Phenotypes of seedlings; (B) fungal biomass and (C) relative expression level of VdAAC determined by qRT-PCR. The bars with different letters (a–e) are significantly different (p < 0.05), based on the Duncan’s multiple range test.
On the basis of the qRT-PCR to estimate fungal biomass in the root, stem and leaves of different groups at 12 dpi (Figure 7B), fungal biomass was significantly lower in transgenic seedlings than in Nb wt. To further examine whether the decreasing disease index in transgenic seedlings resulted from the silencing of VdAAC, we used qRT-PCR to analyze the relative expression level of VdAAC in transgenic and wild-type seedlings (Figure 7C). With the expression level of VdAAC in the Nb wt group estimated as 1, the Trans-1 group had better silencing efficiency (up to 47%) compared with 29% in Trans-2 group. The relative quantitative results, including the level of V. dahliae biomass and VdAAC expression, were strongly in accordance with the phenotypes of different groups.
4. Discussion
Because the membrane protein AAC is needed to maintain a balance between ADP and ATP to generate energy for cells, we postulated that siRNAs designed against the VdAAC gene could be introduced into V. dahliae to decrease the expression of VdAAC; silencing of VdAAC could ultimately inhibit the mycelial growth and sporulation. By transforming fungal protoplasts in this way, we confirmed this hypothesis. Further support for this data comes from a previous study in which the silencing of a single functional AAC gene (TbAAC), in Trypanosoma brucei, by RNAi resulted in a severe growth defect, mainly due to reduced mitochondrial ATP synthesis [28]. Consistent with our RNAi results, the ΔVdAAC mutant had significant reduced colony diameter, conidia number and virulence as compared with Vd wt and ΔVdAAC-C.
Previous studies indicated that genes related to energy metabolism are upregulated in response to adverse environments [52,53,54]. In litchi fruit, when exposed to cold temperature, short-term anaerobic and pure oxygen conditions, genes related to energy metabolism including LcAAC were found to be upregulated [55]. Under reduced oxygen tension, the AAC gene deletion mutants of Schizosaccharomyces pombe exhibited impaired growth and were also unable to grow on a nonfermentable carbon source [56]. In our study, however, we found that VdAAC gene does not have similar role of adapting the fungus to adverse conditions. When exposed to UV light and high osmotic stress, the ratio of reduction in mycelial growth and sporulation for ΔVdAAC with Vd wt and ΔVdAAC-C strains were not significant from no stress conditions. Overall, the gene deletion mutants (ΔVdAAC) grows at roughly 60% of the wild type (Vd wt) and complementary strains (ΔVdAAC-C) rate under both stress and no stress conditions.
The AAC gene has a vital role in maintaining ADP/ATP balance in vivo, transporting ATP to the cytoplasm and ADP to mitochondria [57,58]. The movement of adenine-nucleotide (ADP/ATP) across the inner membrane of mitochondria is dependent on the concentration of internal ATP and on the energy state of mitochondria. The knockout of this gene can have a significant effect on genes that are putatively involved in energy metabolism e.g., vacuolar ATPase (VDAG_05626.1, VdVA) was upregulated in gene deletion mutants. The deletion of AAC gene also had a significant impact on the expression ATP synthase F0 subunit 6 (VDAG_17005.1, VdATP6). Similarly adenylate cyclase (VDAG_04508.1, VdAC) and ATP phosphoribosyltransferase (VDAG_08760.1, VdATP-PRT), were also upregulated in mutants as compared to the wild type and complementary strains.
Under favorable conditions, conidia germinate and produce a germ tube as the first step to invade a host and initiate wilt disease [59]. As we discussed earlier, sporulation is also a requisite factor for this fungus to infect the host [60]. The genes involved in germination and sporulation have become a target to control this fungus [61]. Sporulation is significantly impaired in VdPR3 deletion mutants, which had decreased virulence on cotton [62]. The disruption of VdRNS/ER downregulated glycan synthesis, leading to the inhibition of conidia germination and infection by V. dahliae [63]. In this study, germination and sporulation were significantly reduced in ΔVdAAC, suggesting these reductions were the main reason for reduced fungal biomass and virulence in Nb wt.
Transgenic plants harboring dsRNA against appropriate target genes can have improved resistance [64,65]. Expression of 16D10 dsRNA in Arabidopsis improved resistance against the four major species of root-knot nematodes [66]. Transgenic tomato plants expressing a hairpin construct exhibited resistance against potato spindle tuber viroid infection [67]. Wheat plants transformed with RNAi constructs against three targeted fungal genes exhibited strong resistance against Puccinia triticina and thus a suppressed disease phenotype [36]. In our study, in the transgenic lines of N. benthamiana that expressed dsVdAAC, expression of the targeted gene and fungal biomass were reduced in the plant, which also had a lower disease index than Nb wt did.
5. Conclusions
In this study, the siRNAs transformed into V. dahliae protoplasts silenced VdAAC, and mycelial growth and sporulation were inhibited. Gene knockout mutants, as compared with wild-type and complementary strains, were impaired in mycelial growth, conidia production, stress tolerance and virulence. Moreover, the transgenic plants expressing dsVdAAC showed enhanced resistance against V. dahliae. Taken together, the present data demonstrates that VdAAC has potential as a target gene for an RNAi-based strategy to protect crops against V. dahliae.
Acknowledgments
This work was funded by a grant from National Nonprofit Industry Research (201503109).
Abbreviations
The following abbreviations are used in this manuscript:
| AAC | ADP, ATP carrier |
| siRNAs | short interfering RNAs |
| RNAi | RNA interference |
| CM | complete medium broth |
| MS | Murashige-Skoog |
| dpi | days post inoculation |
| DI | disease index |
| ORF | open reading frame |
Supplementary Materials
The following are available online at www.mdpi.com/2073-4425/8/1/25/s1, Figure S1: Position of siRNAs designed from different regions of the VdAAC gene of V. dahliae and colony diameter in different RNAi-treated groups. (A) Position of siRNAs along the VdAAC gene. siRNAs were designed and synthesized by Oligobio, Beijing, China; (B) Colony diameters of control and siRNA groups observed 2 weeks after transformation on PDA agar plates. Figure S2: Evaluation of resistance for wild-type (Nb wt) and transgenic N. benthamiana against V. dahliae. (A) Region (189–836 bp) of VdAAC gene was amplified and cloned into pK7GW1WG2(I) by LR recombination reaction. Numbers indicate nucleotide positions; (B) Schematic representation of the pK7GWIWG2(I)-VdAAC construction containing the sense and antisense partial ORF of VdAAC; (C) Confirmation of transgenic plants transformed with pK7GWIWG2(I)-VdAAC by PCR. The Nb wt seedling served as the negative control. (D) Disease index for seedlings from 10 to 12 days post inoculation with V. dahliae. Figure S3: Virulence and germination analysis of mutants and wild-type V. dahliae (Vd wt). (A) Disease index for N. benthamiana seedlings at 10 to 12 dpi with ΔVdAAC, ΔVdAAC-C and Vd wt; (B) Percentage germination of conidia produced by Vd-GFP or ΔVdAAC-GFP after 48 h on PDA.
Author Contributions
Hongmei Cheng, Rui Zhang and Huiming Guo conceived and designed the experiments. Xiaofeng Su, Latifur Rehman and Xiaokang Li performed experiments and analysed data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Klosterman S.J., Atallah Z.K., Vallad G.E., Subbarao K.V. Diversity, pathogenicity, and management of Verticillium species. Annu. Rev. Phytopathol. 2009;47:39–62. doi: 10.1146/annurev-phyto-080508-081748. [DOI] [PubMed] [Google Scholar]
- 2.Pang J., Zhu Y., Li Q., Liu J., Tian Y., Liu Y., Wu J. Development of Agrobacterium-mediated virus-induced gene silencing and performance evaluation of four marker genes in Gossypium barbadense. PLoS ONE. 2013;8:e73211. doi: 10.1371/journal.pone.0073211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pegg G.F., Brady B.L. Verticillium Wilts. CABI Publishing; Oxford, UK: 2002. [Google Scholar]
- 4.Wang Y., Liang C., Wu S., Zhang X., Tang J., Jian G., Jiao G., Li F., Chu C. Significant improvement of cotton Verticillium wilt resistance by manipulating the expression of gastrodia antifungal proteins. Mol. Plant. 2016;9:1436–1439. doi: 10.1016/j.molp.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Tsror L., Levin A.G. Vegetative compatibility and pathogenicity of Verticillium dahliae Kleb. Isolates from Olive in Israel. J. Phytopathol. 2003;151:451–455. doi: 10.1046/j.1439-0434.2003.00749.x. [DOI] [Google Scholar]
- 6.Duressa D., Anchieta A., Chen D., Klimes A., Garcia-Pedrajas M.D., Dobinson K.F., Klosterman S.J. RNA-seq analyses of gene expression in the microsclerotia of Verticillium dahliae. BMC Genom. 2013;14:607. doi: 10.1186/1471-2164-14-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fradin E.F., Thomma B.P. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006;7:71–86. doi: 10.1111/j.1364-3703.2006.00323.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D.D., Wang X.Y., Chen J.Y., Kong Z.Q., Gui Y.J., Li N.Y., Bao Y.M., Dai X.F. Identification and characterization of a pathogenicity-related gene VdCYP1 from Verticillium dahliae. Sci. Rep. 2016 doi: 10.1038/srep27979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong D., Wang Y., Tang C., Fang Y., Zou J., Tian C. VdCrz1 is involved in microsclerotia formation and required for full virulence in Verticillium dahliae. Fungal Genet. Biol. 2015;82:201–212. doi: 10.1016/j.fgb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Tian L., Xu J., Zhou L., Guo W. VdMsb regulates virulence and microsclerotia production in the fungal plant pathogen Verticillium dahliae. Gene. 2014;550:238–244. doi: 10.1016/j.gene.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Nakayashiki H., Hanada S., Nguyen B.Q., Kadotani N., Tosa Y., Mayama S. RNA silencing as a tool for exploring gene function in ascomycete fungi. Fungal Genet. Biol. 2005;42:275–283. doi: 10.1016/j.fgb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Deshmukh R., Purohit H.J. siRNA mediated gene silencing in Fusarium sp. HKF15 for overproduction of bikaverin. Bioresour. Technol. 2014;157:368–371. doi: 10.1016/j.biortech.2014.02.057. [DOI] [PubMed] [Google Scholar]
- 13.Mumbanza F.M., Kiggundu A., Tusiime G., Tushemereirwe W.K., Niblett C., Bailey A. In vitro antifungal activity of synthetic dsRNA molecules against two pathogens of banana, Fusarium oxysporum f. sp. cubense and Mycosphaerella fijiensis. Pest Manag. Sci. 2013;69:1155–1162. doi: 10.1002/ps.3480. [DOI] [PubMed] [Google Scholar]
- 14.Singh S., Braus-Stromeyer S.A., Timpner C., Tran V.T., Lohaus G., Reusche M., Knufer J., Teichmann T., von Tiedemann A., Braus G.H. Silencing of Vlaro2 for chorismate synthase revealed that the phytopathogen Verticillium longisporum induces the cross-pathway control in the xylem. Appl. Microbiol. Biotechnol. 2010;85:1961–1976. doi: 10.1007/s00253-009-2269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch A., Kumar N., Weber L., Keller H., Imani J., Kogel K.H. Host-induced gene silencing of cytochrome P450 lanosterol C14alpha-demethylase-encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA. 2013;110:19324–19329. doi: 10.1073/pnas.1306373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghag S.B., Shekhawat U.K., Ganapathi T.R. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol. J. 2014;12:541–553. doi: 10.1111/pbi.12158. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T., Jin Y., Zhao J.H., Gao F., Zhou B.J., Fang Y.Y., Guo H.S. Host-induced gene silencing of target gene in fungal cells confers effective resistance to cotton wilt disease pathogen Verticillium dahliae. Mol. Plant. 2016;9:939–942. doi: 10.1016/j.molp.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Nury H., Dahout-Gonzalez C., Trezeguet V., Lauquin G., Brandolin G., Pebay-Peyroula E. Structural basis for lipid-mediated interactions between mitochondrial ADP/ATP carrier monomers. FEBS Lett. 2005;579:6031–6036. doi: 10.1016/j.febslet.2005.09.061. [DOI] [PubMed] [Google Scholar]
- 19.Klingenberg M. Molecular aspects of the adenine nucleotide carrier from mitochondria. Arch. Biochem. Biophys. 1989;270:1–14. doi: 10.1016/0003-9861(89)90001-5. [DOI] [PubMed] [Google Scholar]
- 20.Fiore C., Le S.A., Roux P., Schwimmer C., Dianoux A.N.F., Gjm L., Brandolin G., Pv V., Trezeguet V. The mitochondrial ADP/ATP carrier: Structural, physiological and pathological aspects. Biochimie. 1998;80:137–150. doi: 10.1016/S0300-9084(98)80020-5. [DOI] [PubMed] [Google Scholar]
- 21.Pebay-Peyroula E., Dahout-Gonzalez C., Kahn R., Trezeguet V., Lauquin G.J., Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- 22.Hatanaka T., Kihira Y., Shinohara Y., Majima E., Terada H. Characterization of loops of the yeast mitochondrial ADP/ATP carrier facing the cytosol by site-directed mutagenesis. Biochem. Biophys. Res. Commun. 2001;286:936–942. doi: 10.1006/bbrc.2001.5498. [DOI] [PubMed] [Google Scholar]
- 23.Ohkura K., Hori H., Shinohara Y. Role of C-terminal region of yeast ADP/ATP carrier 2 protein: Dynamics of flexible C-terminal arm. Anticancer Res. 2009;29:4897–4900. [PubMed] [Google Scholar]
- 24.Nowara D., Gay A., Lacomme C., Shaw J., Ridout C., Douchkov D., Hensel G., Kumlehn J., Schweizer P. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell. 2010;22:3130–3141. doi: 10.1105/tpc.110.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira C., Chaves S., Alves S., Salin B., Camougrand N., Manon S., Sousa M.J., Corte-Real M. Mitochondrial degradation in acetic acid-induced yeast apoptosis: The role of Pep4 and the ADP/ATP carrier. Mol. Microbiol. 2010;76:1398–1410. doi: 10.1111/j.1365-2958.2010.07122.x. [DOI] [PubMed] [Google Scholar]
- 26.Gigolashvili T., Geier M., Ashykhmina N., Frerigmann H., Wulfert S., Krueger S., Mugford S.G., Kopriva S., Haferkamp I., Flugge U.I. The Arabidopsis thylakoid ADP/ATP carrier TAAC has an additional role in supplying plastidic phosphoadenosine 5’-phosphosulfate to the cytosol. Plant Cell. 2012;24:4187–4204. doi: 10.1105/tpc.112.101964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talbot D.A., Duchamp C., Rey B., Hanuise N., Rouanet J.L., Sibille B., Brand M.D. Uncoupling protein and ATP/ADP carrier increase mitochondrial proton conductance after cold adaptation of king penguins. J. Physiol. 2004;558:123–135. doi: 10.1113/jphysiol.2004.063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gnipova A., Subrtova K., Panicucci B., Horvath A., Lukes J., Zikova A. The ADP/ATP carrier and its relationship to oxidative phosphorylation in ancestral protist Trypanosoma brucei. Eukaryot. Cell. 2015;14:297–310. doi: 10.1128/EC.00238-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rehman L., Su X., Guo H., Qi X., Cheng H. Protoplast transformation as a potential platform for exploring gene function in Verticillium dahliae. BMC Biotechnol. 2016;16:57–65. doi: 10.1186/s12896-016-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H.M., Lin Z.X., Zhang X.L., Chen W., Guo X.P., Nie Y.C., Li Y.H. Mapping and quantitative trait loci analysis of Verticillium wilt resistance genes in cotton. J. Integr. Plant Biol. 2008;50:174–182. doi: 10.1111/j.1744-7909.2007.00612.x. [DOI] [PubMed] [Google Scholar]
- 31.Tian J., Zhang X., Liang B., Li S., Wu Z., Wang Q., Leng C., Dong J., Wang T. Expression of baculovirus anti-apoptotic genes p35 and op-iap in cotton (Gossypium hirsutum L.) enhances tolerance to Verticillium wilt. PLoS ONE. 2010;5:e14218. doi: 10.1371/journal.pone.0014218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X., Ben S., Sun Y., Fan X., Tian C., Wang Y. Genome-wide identification, phylogeny and expression profile of vesicle fusion components in Verticillium dahliae. PLoS ONE. 2013;8:e68681. doi: 10.1371/journal.pone.0068681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Biochem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 34.Hoppenau C.E., Tran V.-T., Kusch H., Aßhauer K.P., Landesfeind M., Meinicke P., Popova B., Braus-Stromeyer S.A., Braus G.H. Verticillium dahliae VdTHI4, involved in thiazole biosynthesis, stress response and DNA repair functions, is required for vascular disease induction in tomato. Environ. Exp. Bot. 2014;108:14–22. doi: 10.1016/j.envexpbot.2013.12.015. [DOI] [Google Scholar]
- 35.Tzima A.K., Paplomatas E.J., Tsitsigiannis D.I., Kang S. The G protein beta subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae. Fungal Genet. Biol. 2012;49:271–283. doi: 10.1016/j.fgb.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Panwar V., McCallum B., Bakkeren G. Endogenous silencing of Puccinia triticina pathogenicity genes through in planta-expressed sequences leads to the suppression of rust diseases on wheat. Plant J. 2013;73:521–532. doi: 10.1111/tpj.12047. [DOI] [PubMed] [Google Scholar]
- 37.Ellendorff U., Fradin E.F., de Jonge R., Thomma B.P. RNA silencing is required for Arabidopsis defence against Verticillium wilt disease. J. Exp. Bot. 2009;60:591–602. doi: 10.1093/jxb/ern306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z., Song Y., Liu C.M., Thomma B.P. Mutational analysis of the Ve1 immune receptor that mediates Verticillium resistance in tomato. PLoS ONE. 2014;9:e99511. doi: 10.1371/journal.pone.0099511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao Y., Cai W., Wang J., Hong G., Tao X., Wang L., Huang Y., Chen X. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007;25:1307–1313. doi: 10.1038/nbt1352. [DOI] [PubMed] [Google Scholar]
- 40.Obrepalska-Steplowska A., Wieczorek P., Budziszewska M., Jeszke A., Renaut J. How can plant virus satellite RNAs alter the effects of plant virus infection? A study of the changes in the Nicotiana benthamiana proteome after infection by peanut stunt virus in the presence or absence of its satellite RNA. Proteomics. 2013;13:2162–2175. doi: 10.1002/pmic.201200056. [DOI] [PubMed] [Google Scholar]
- 41.Klionsky D.J., Nelson H., Nelson N., Yaver K. Mutations in the yeast vacuolar ATPase result in the mislocalization of vacuolar proteins. J. Exp. Biol. 1992;172:83–92. doi: 10.1242/jeb.172.1.83. [DOI] [PubMed] [Google Scholar]
- 42.Clague M.J., Urbe S., Aniento F., Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- 43.Klionsky D.J., Herman P.K., Emr S.D. The fungal vacuole: Composition, function, and biogenesis. Microbiol. Rev. 1990;54:266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deckers-Hebestreit G., Schmid R., Kiltz H.H., Altendorf K. F0 portion of Escherichia coli ATP synthase: Orientation of subunit c in the membrane. Biochemistry. 1987;26:5486–5492. doi: 10.1021/bi00391a041. [DOI] [PubMed] [Google Scholar]
- 45.Van Walraven H.S., Scholts M.J., Lill H., Matthijs H.C., Dilley R.A., Kraayenhof R. Introduction of a carboxyl group in the loop of the F0 c-subunit affects the H+/ATP coupling ratio of the ATP synthase from Synechocystis 6803. J. Bioenerg. Biomembr. 2002;34:445–454. doi: 10.1023/A:1022566025300. [DOI] [PubMed] [Google Scholar]
- 46.Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal. Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-X. [DOI] [PubMed] [Google Scholar]
- 47.Klimpel A., Gronover C.S., Williamson B., Stewart J.A., Tudzynski B. The adenylate cyclase (BAC) in Botrytis cinerea is required for full pathogenicity. Mol. Plant Pathol. 2002;3:439–450. doi: 10.1046/j.1364-3703.2002.00137.x. [DOI] [PubMed] [Google Scholar]
- 48.Piszkiewicz D., Tilley B.E., Rand-Meir T., Parsons S.M. Amino acid sequence of ATP phosphoribosyltransferase of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA. 1979;76:1589–1592. doi: 10.1073/pnas.76.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho Y., Sharma V., Sacchettini J.C. Crystal structure of ATP phosphoribosyltransferase from Mycobacterium tuberculosis. J. Biol. Chem. 2003;278:8333–8339. doi: 10.1074/jbc.M212124200. [DOI] [PubMed] [Google Scholar]
- 50.Brenner M., Ames B.N. The histidine operon and its regulation. In: Vogel H.J., editor. Metabolic Regulation. Volume 5. Academic Press; New York, NY, USA: 1971. pp. 349–387. [Google Scholar]
- 51.Goldberger R.F., Kovach J.S. Regulation of histidine biosynthesis in Salmonella typhimurium. Curr. Top. Cell. Regul. 1972;5:285–308. doi: 10.1016/b978-0-12-152805-8.50014-9. [DOI] [PubMed] [Google Scholar]
- 52.Voncken F., Gao F., Wadforth C., Harley M., Colasante C. The phosphoarginine energy-buffering system of Trypanosoma brucei involves multiple arginine kinase isoforms with different subcellular locations. PLoS ONE. 2013;8:e65908. doi: 10.1371/journal.pone.0065908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pereira C.A. Arginine kinase: A potential pharmacological target in trypanosomiasis. Infect. Disord. Drug Targets. 2014;14:30–36. doi: 10.2174/1871526514666140713144103. [DOI] [PubMed] [Google Scholar]
- 54.Miranda M.R., Canepa G.E., Bouvier L.A., Pereira C.A. Trypanosoma cruzi: Oxidative stress induces arginine kinase expression. Exp. Parasitol. 2006;114:341–344. doi: 10.1016/j.exppara.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Liu T., Wang H., Kuang J., Sun C., Shi J., Duan X., Qu H., Jiang Y. Short-term anaerobic, pure oxygen and refrigerated storage conditions affect the energy status and selective gene expression in litchi fruit. LWT-Food Sci. Technol. 2015;60:1254–1261. doi: 10.1016/j.lwt.2014.09.003. [DOI] [Google Scholar]
- 56.Trezeguet V., Zeman I., David C., Lauquin G.J., Kolarov J. Expression of the ADP/ATP carrier encoding genes in aerobic yeasts; phenotype of an ADP/ATP carrier deletion mutant of Schizosaccharomyces pombe. Biochim. Biophys. Acta. 1999;1410:229–236. doi: 10.1016/S0005-2728(98)00180-7. [DOI] [PubMed] [Google Scholar]
- 57.Miura K., Inouye S., Sakai K., Takaoka H., Kishi F., Tabuchi M., Tanaka T., Matsumoto H., Shirai M., Nakazawa T., Nakazawa A. Cloning and characterization of adenylate kinase from Chlamydia pneumoniae. J. Biol. Chem. 2001;276:13490–13498. doi: 10.1074/jbc.M009461200. [DOI] [PubMed] [Google Scholar]
- 58.Claypool S.M., Oktay Y., Boontheung P., Loo J.A., Koehler C.M. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 2008;182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hawke M.A., Lazarovits G. Production and manipulation of individual microsclerotia of Verticillium dahliae for use in studies of survival. Phytopathology. 1994;23:582–584. [Google Scholar]
- 60.Isaac I. Verticillium wilt of sainfoin. Ann. Appl. Biol. 1946;33:28–34. doi: 10.1111/j.1744-7348.1946.tb06269.x. [DOI] [PubMed] [Google Scholar]
- 61.Debode J., De Maeyer K., Perneel M., Pannecoucque J., De Backer G., Hofte M. Biosurfactants are involved in the biological control of Verticillium microsclerotia by Pseudomonas spp. J. Appl. Microbiol. 2007;103:1184–1196. doi: 10.1111/j.1365-2672.2007.03348.x. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y.L., Li Z.F., Feng Z.L., Feng H.J., Zhao L.H., Shi Y.Q., Hu X.P., Zhu H.Q. Isolation and functional analysis of the pathogenicity-related gene VdPR3 from Verticillium dahliae on cotton. Curr. Genet. 2015;61:555–566. doi: 10.1007/s00294-015-0476-z. [DOI] [PubMed] [Google Scholar]
- 63.Santhanam P., Boshoven J.C., Salas O., Bowler K., Islam T., Keykha Saber M., van den Berg G.C., Bar-Peled M., Thomma B.P. Rhamnose synthase activity is required for pathogenicity of the vascular wilt fungus Verticillium dahliae. Mol. Plant Pathol. 2016 doi: 10.1111/mpp.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novina C.D., Sharp P.A. The RNAi revolution. Nature. 2004;430:161–164. doi: 10.1038/430161a. [DOI] [PubMed] [Google Scholar]
- 65.Kalantidis K., Psaradakis S., Tabler M., Tsagris M. The occurrence of CMV-specific short RNAS in transgenic tobacco expressing virus-derived double-stranded RNA is indicative of resistance to the virus. MPMI. 2002;15:826–833. doi: 10.1094/MPMI.2002.15.8.826. [DOI] [PubMed] [Google Scholar]
- 66.Huang G., Allen R., Davis E.L., Baum T.J., Hussey R.S. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. USA. 2006;103:14302–14306. doi: 10.1073/pnas.0604698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwind N., Zwiebel M., Itaya A., Ding B., Wang M.B., Krczal G., Wassenegger M. RNAi-mediated resistance to Potato spindle tuber viroid in transgenic tomato expressing a viroid hairpin RNA construct. Mol. Plant Pathol. 2009;10:459–469. doi: 10.1111/j.1364-3703.2009.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.