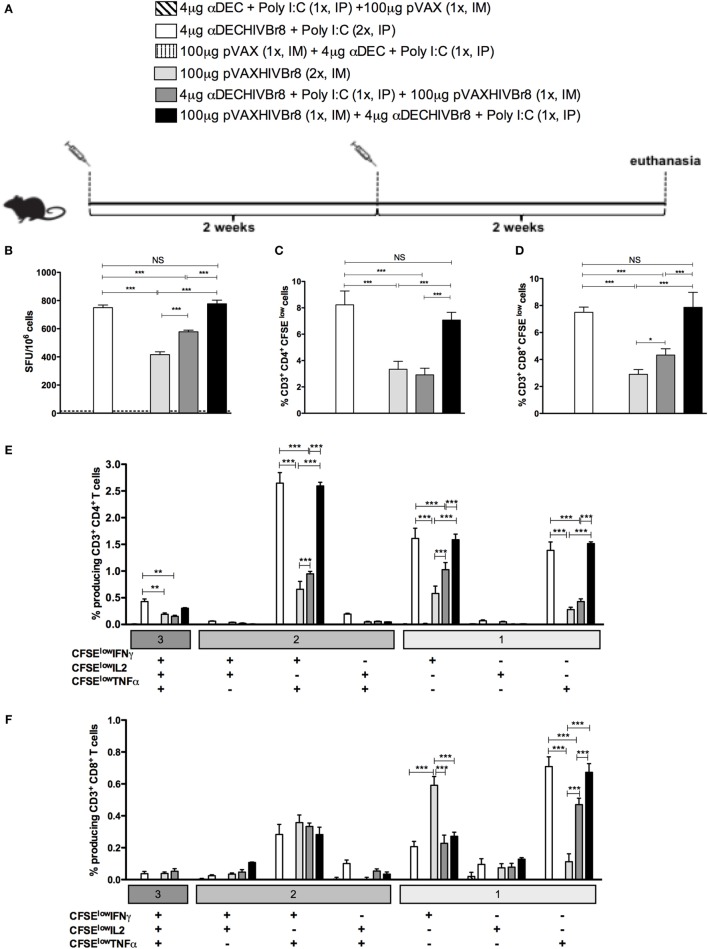
Figure 4.
Homologous αDECHIVBr8 monoclonal antibody (mAb) immunization or heterologous prime-boost induces polyfunctional T cell responses. BALB/c mice (n = 6) were immunized with two doses of 4 µg of αDECHIVBr8 in the presence of poly (I:C) adjuvant (IP) or two doses of 100 µg of pVAXHIVBr8 DNA vaccine (IM). For heterologous regimens, mice were immunized with one dose of αDECHIVBr8 followed by one dose of DNA vaccine or vice versa. The control groups were immunized with one dose of αDEC mAb together with poly (I:C) followed by one dose with pVAX or vice versa. (A) Immunization scheme. Fifteen days after the second dose, the spleen of each animal was removed and the splenocytes (B) were cultured in the presence of pooled HIV-1 peptides (5 µM) for 18 h to evaluate the number of IFN-γ-producing cells by ELISpot assay. SFU, spot forming units. Cutoff = 15 SFU/106 cells and is represented by the dotted line. (C,D) Splenocytes were labeled with carboxyfluorescein succinimidyl ester (CFSE) (1.25 µM) and cultured in the presence of pooled HIV-1 peptides (5 µM) for 5 days to evaluated specific proliferation. After staining with fluorochrome-labeled anti-CD3, anti-CD4, and anti-CD8 monoclonal antibodies, cells were analyzed by flow cytometry. CFSE dilution on gated CD3+CD4+ (C) or CD3+CD8+ (D) cells was used as readout for antigen-specific proliferation; For detection of cytokine-producing T cells, cells were pulsed on day 4 for 12 h with pooled peptides in the presence of anti-CD28 and brefeldin A. Cells were then surface stained with anti-CD3, CD4, and CD8, permeabilized, and stained for intracellular cytokines. Multiparameter flow cytometry was used to determine the frequency of IFNγ-, IL2-, or TNFα-producing CD4+ and CD8+ T cells (E,F). After gating on proliferating (CFSElow) and cytokine-producing cells, Boolean combinations were then created using FlowJo software to determine the frequency of each response based on all possible combinations of cytokine-producing CD4+ (E) and CD8+ (F) T cells. One million events were acquired in a live lymphocyte gate. The percentage of proliferating and cytokine-producing T cells was calculated subtracting the values of stimulated from non-stimulated cultures. NS, not significant; *p < 0.05; **p < 0.01; ***p < 0.001. Data represent mean ± SD.