Figure 6.
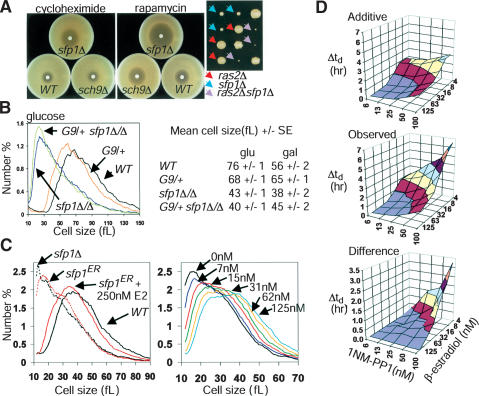
Sfp1 and Sch9 function in parallel pathways. (A) sfp1Δ cells are sensitive to cycloheximide and to decreases in TOR or PKA pathway activity. (Left) Filter disks containing 3 nmole cycloheximide or 15 μg rapamycin were incubated on lawns of the indicated strains for 2 d. (Right) Synthetic proliferation defects between ras2Δ and sfp1Δ. Spore clones were scored after 4 d. (B) Sch9 does not control cell size strictly via Sfp1. Size distributions of an sfp1Δ/Δ strain in the presence or absence of a functionally null heterozygous GAL1–SCH9 (G9/+) allele are shown. Mean cell sizes of the indicated strains in either rich glucose or galactose medium are indicated to the right (n = 4). (C) An sfp1ER allele is modulated by β-estradiol (E2). Size distributions of log phase sfp1ER cultures with or without 250 nM E2 (left) or in the presence of various E2 concentrations (right). (D) Progressively compromised SCH9 and SFP1 activity reveals synergistic proliferation defects. An sch9as sfp1ER strain was inoculated into varying concentrations of 1NM-PP1 and E2 in synthetic glucose medium. Doubling times (td) were determined for each culture and the increase relative to cultures proliferating in 6.25 nM 1NM-PP1 and 125 nM E2 (concentrations at which Sch9as and Sfp1ER were fully active) was calculated. (Top) The increase in doubling time (Δtd) resulting from individually increasing 1NM-PP1 or decreasing E2 was used to calculate the predicted additive effects. Actual increases in doubling time (middle) and the difference (bottom) are plotted.