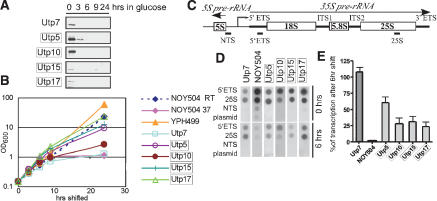
Figure 3.
Transcription run-on analysis indicates that depletion of a subgroup of SSU processome components leads to a reduction in pre-rRNA transcription. Each strain carries the single gene encoding the indicated protein under the control of a GAL promoter (GAL::HA-UTP), allowing for conditional expression. The boxes indicate proteins whose depletion affects levels of all pre-rRNAs (from Fig. 2), and that were therefore hypothesized to be affecting transcription. The NOY504 strain carries a temperature-sensitive RNA polymerase I. (A) The Utp proteins are depleted when the GAL::HA-UTP strains are grown in glucose. Western blots with anti-HA antibody were performed on protein harvested from yeast expressing the indicated tagged proteins under the control of a GAL promoter prior to (0) or after (3, 6, 9, 24 h) the switch to glucose. (B) Growth curves of yeast strains. Growth of the GAL::HA-UTP and the parent YPH499 strains in glucose is indicated graphically and compared to growth of the NOY504 strain at the permissive (room temperature [RT]) and nonpermissive (37°C) temperatures. (C) The rDNA transcription unit. RNA polymerase I transcribes the 35S primary rRNA transcript, whereas RNA polymerase III transcribes the 5S rRNA in the opposite direction. PCR products corresponding to the indicated segments of the nontranscribed spacer (NTS), the 5′ external transcribed spacer (5′ETS), and the 25S rRNA were cloned into the pTOPO plasmid. (D) Transcription run-on analysis. The GAL::HA-UTP strains were either not depleted or depleted for 6 h of the indicated proteins, permeabilized, and exposed to α32P-UTP for 10 min. RNA was harvested and hybridized to plasmids described in C dot-blotted onto Hybond-N+ membrane. For the NOY504 strain, cultures were incubated at 37°C for 6 h. (E) Quantitation of the results in D.