Figure 6.
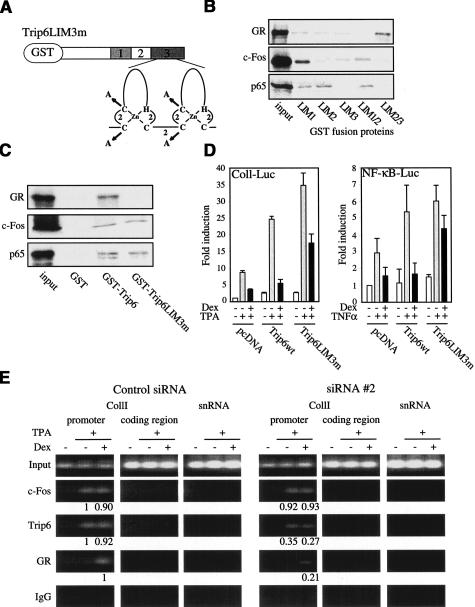
nTrip6 mediates GR tethering to the repressed promoter. (A) Schematic representation of Trip6LIM3m fused to GST. Trip6 harbors 3 LIM domains in its C-terminal patch, each consisting of two zinc fingers, as illustrated for the LIM domain 3 (LIM3). In Trip6LIM3m, the four coordinating cysteines in the two zinc fingers of LIM3 were mutated to alanines. (B) Mapping of Trip6 LIM domains interaction with GR, c-Fos, and NF-κB p65. 35S-labeled, in vitro translated GR, c-Fos, and p65 were subjected to GST pull-down assays using GST fusion of individual Trip6 LIM domains, or combinations of the LIM domains 1 and 2, or 2 and 3. Input represents 10% of the in vitro translated material used in each assay. (C) Trip6LIM3m does not interact with GR, but still interacts with c-Fos and NF-κB p65. GST pull-down assays were performed as in B using GST or GST fusions of Trip6 or Trip6LIM3m. (D) Trip6LIM3m has a dominant negative effect on AP-1 and NF-κB repression by GR. HEK-293 (left) or A549 cells (right) were cotransfected with the indicated luciferase reporter construct and expression vectors. Cells were treated with TPA (left) or TNFα (right), in the presence or absence of dexamethasone (Dex). Normalized luciferase activities (to renilla luciferase) are plotted as fold induction (mean ± S.D. of one representative experiment performed in triplicates). (E) nTrip6 mediates the tethering of GR to the repressed collagenase I promoter. Chromatin immunoprecipitation (ChIP) was performed as in Figure 5 in HeLa cells transfected with either the control siRNA or the siRNA #2, and treated with TPA in the presence or absence of Dex as indicated. The relative promoter occupancy by c-Fos, nTrip6, and GR (normalized to the input) is indicated below the bands.