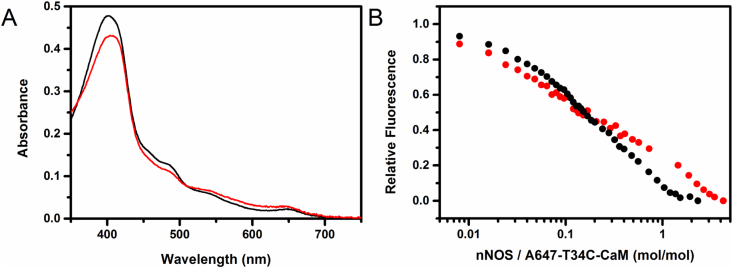
Fig. 3.
Redox-dependent binding of CaM to nNOS. A) shows the UV–visible absorbance spectra of oxidised (black) and dithionite-reduced (red) nNOS (∼5 μM). The dithionite reduced NOS was shown to be stable for over 2 h under air and the absorbance is not perturbed by the addition of a 5-fold excess of T34C-CaM (data not shown). B) shows the titration of 5 nM A647-T34C-CaM with oxidised (black) and dithionite-reduced (red) nNOS monitored by quenching of the fluorescence emission from the A647 bound to CaM. All materials were of analytic grade and purchased from Sigma-Aldrich, except Alexa Fluor 647 C2 maleimide (A647)m which was purchased from Thermofisher scientific. Both recombinant rat neuronal nitric oxide synthase and the T34C-CaM variant were expressed and purified as previously described [43], [69], [78], [79]. Purified nNOS was oxidised with ferricyanide and passed down a desalting column to remove excess oxidising agent. To reduce the oxidised nNOS, excess sodium dithionite mixed with the oxidised for of nNOS, which was subsequently passed down a desalting column equilibrated with the buffering solution. A647 maleimide was bound to T34C-CaM in the dark using previously published protocols [43]. Fluorescence measurements were made with an Edinburgh Instruments (Livingston, UK) FLS920 fluorometer. Emission spectra were taken using 5 nm excitation and 5 nm emission slit-widths in 1 mL fluorescent quartz cells (Starna Scientific Ltd, Hainault, UK) with a 10 mm excitation path length. Data were collected at room temperature in 40 mM HEPES (pH 7.6), 150 mM NaCl,1 mM CaCl2 and 10% glycerol. Under these conditions, no fluorophore photo-bleaching was observed.