Figure 5.
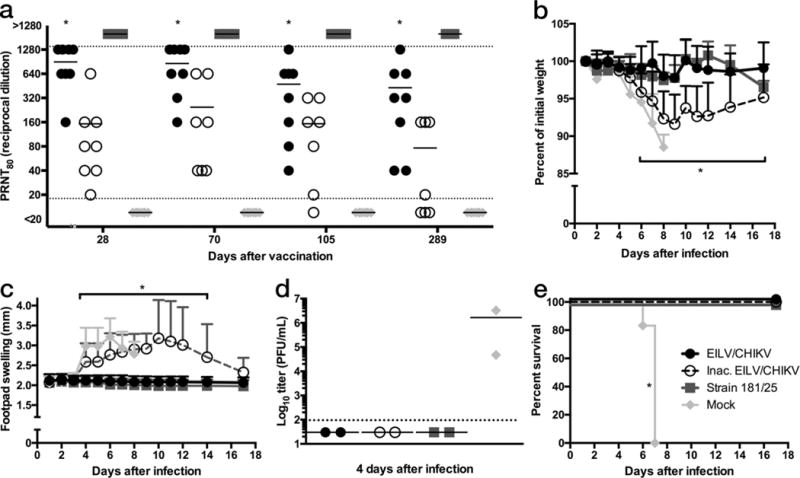
A single-dose of EILV/CHIKV is immunogenic and provides long-term protection in a lethal, immunocompromised model of CHIKV-infection. IFNα/βR−/− mice (n=8/group) were vaccinated with EILV/CHIKV, formalin-inactivated EILV/CHIKV, live-attenuated strain 181/clone25, or mock vaccinated and (a) 80% plaque-reduction neutralizing antibody titers (PRNT80) were determined at 28, 70, 105, and 289 days post vaccination (DPV) for all 8 animals and each data point and mean value was plotted. (b–e) Four days after challenge with 1×103 PFU of CHIKV-99650 ID in the rear footpad, three mice per group were sacrificed and blood and pelvic limbs were harvested for (d) viremia and histopathological analyses (Supplementary Fig. 7), respectively. The remaining five mice per group were utilized for analysis of (b) weight change, (c) footpad swelling, and (e) survival at the indicated time points with (b,c) represented as mean + s.d.. (d) Blood could not be collected from one mouse so the remaining two are plotted as individual data points as well as the mean value(Statistical analysis: two-way ANOVA with Tukey’s multiple comparison test, (a) EILV/CHIKV compared to all groups * p < 0.05, (b–c) formalin-inactivated EILV/CHIKV and mock * p < 0.05, Fisher’s exact test of final survival proportions, (e) compared to mock * p < 0.05). Experiment performed once.
