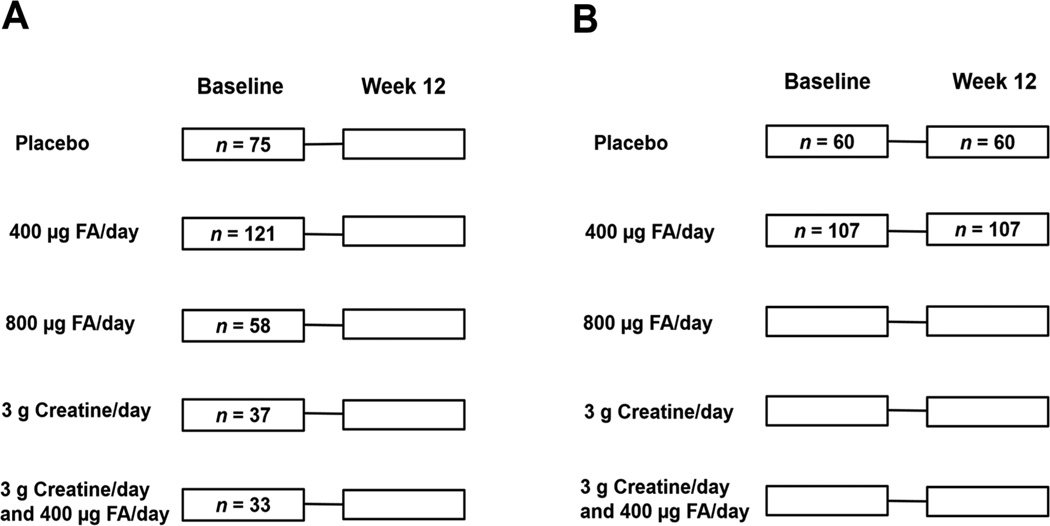
Figure 1. FACT Design and Sampling for Current Study.
A) Participants selected for cross-sectional analyses. Baseline-collected samples from 324 FACT participants were included in cross-sectional analyses for the current study. All participants from the placebo and 400 µg FA/day treatment arms who had complete information for predictors (one-carbon metabolism indices), PTHMs, and potential confounders were included in these analyses (n = 75 for placebo group, n = 121 for 400 µg FA/day group); an additional 128 participants with complete information for all relevant variables were randomly selected from the remaining three treatment arms. The distribution of these participants are shown by treatment arm. B) Participants selected for examination of FA treatment effects. Participants with at least one PTHM measure at both time points of interest (i.e., baseline and week 12) were included in analyses examining the effect of 400 µg FA/day (n = 107) vs placebo (n = 60) on PTHMs.