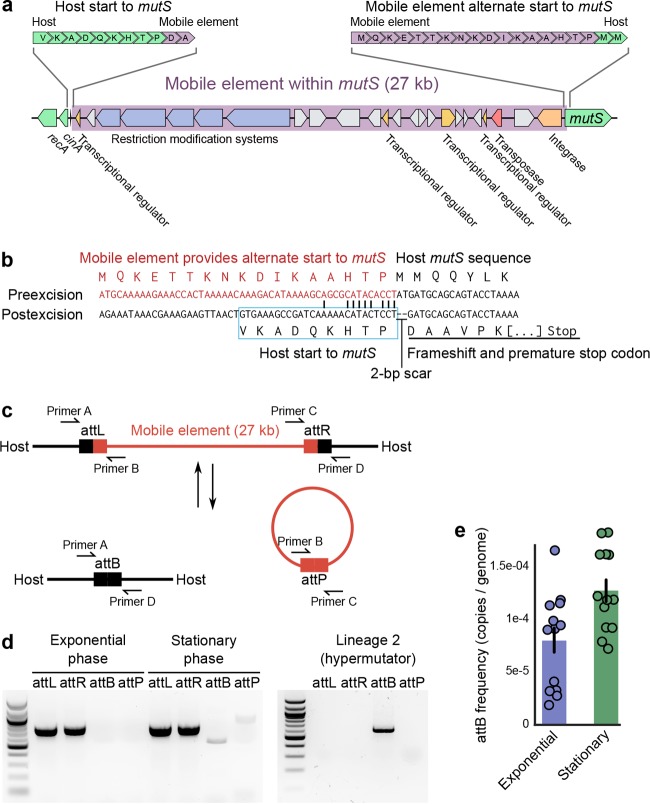
FIG 2 .
Excision of a mobile element within mutS disrupts the mutS genetic sequence. (a) We identified a mobile element adjacent to mutS that was missing in both hypermutator lineages. Further inspection revealed that, when present, the mobile element appeared integrated within the mutS sequence, separating the original host-encoded mutS starting sequence. (b) When integrated, the mobile element provided a new start and upstream regulatory region to the mutS coding sequence. After excision, the mobile element left a 2-bp frameshift deletion in the host’s mutS sequence, resulting in a premature stop codon. (c) We designed a PCR assay to detect the excision of this mobile element. When the mobile element is integrated into the host mutS sequence, the left (attL) and right (attR) attachment site junctions of the mobile element and host genome are amplified. When the mobile element is excised, the rejoined host mutS gene (attB) and the circular excised mobile element (attP) are amplified. Expected amplicon lengths: attL = 819 bp, attR = 836 bp, attB = 613 bp, and attP = 1,042 bp. (d) We found that in rich medium (LB), the mobile element excised itself at low frequency. Sanger sequencing of PCR products attB and attP confirmed the 2-bp frameshift deletion in the host mutS sequence and the transfer of these base pairs to a circularized mobile element. In hypermutator lineages (e.g., lineage 2), we could no longer detect the mobile element, only the scarred host mutS sequence. (e) qPCR assays indicated that the frequency of excision was approximately 1/10,000 genomes, with moderately higher excision frequency during the stationary phase.