FIG 2 .
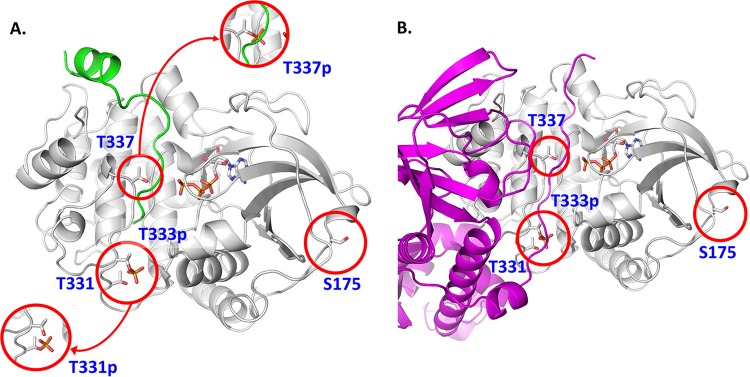
A. fumigatus PKA structural model showing the position of phosphorylated residues that may disrupt PKA activity and function. (A) The A. fumigatus PKA sequence was threaded onto the S. cerevisiae source model (39), with which it shares 48% sequence identity. The backbone of the structure is shown in white, with the bound substrate peptide shown in green and the ATP cofactor depicted in atom colors. Phosphorylation of T331 (T331p, lower inset) would likely preclude phosphorylation of the nearby typically constitutively phosphorylated T333 (T333p) as rendered and would cause structural disruptions to the distal end of the substrate binding site. Phosphorylation of T337 (T337p, upper inset) would cause significant steric hindrance to bound peptides and proteins as well as strong charge repulsion with the triphosphate moiety of the ATP cofactor. (B) The A. fumigatus regulatory protein sequence was threaded onto a Mus musculus source model (40) and is shown in magenta. Part of the N terminus of the regulatory subunit, the “inhibitor sequence,” mimics substrate in the PKA binding site, and as such, transient phosphorylation of T331 and T337 would cause similar structural disruptions, thus breaking important protein-protein contacts in the holoenzyme.