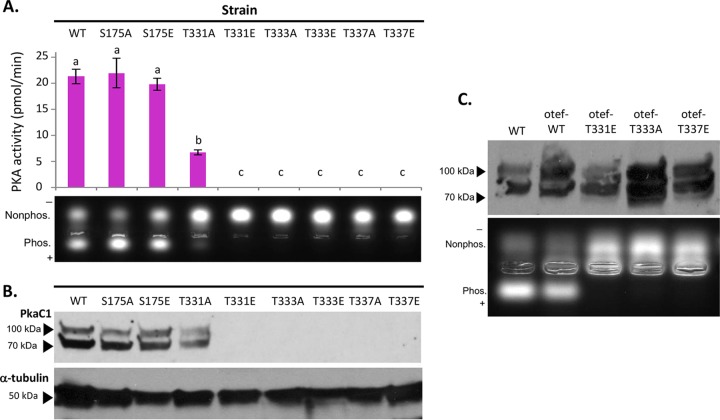
FIG 3 .
Impact of PkaC1 phosphomutations on protein kinase activity. (A) Protein normalized crude cellular extracts for each mutant strain were added in duplicate to reaction mixtures containing fluorescent dye-coupled PepTag peptide (Promega) as a PKA-specific test substrate and incubated for 30 min at room temperature. Activity was assessed qualitatively based on migration of negatively charged, phosphorylated peptide toward the anode of an agarose gel (lower panel) and quantified by comparing the fluorescence of a phosphorylated substrate for each sample to a standard curve using a plate reader, and the average values for each mutant were plotted (upper panel). Activity was defined as picomoles of substrate phosphorylated per minute. Error bars represent standard errors. Activities were analyzed via Student’s t test, and statistically significant differences (P < 0.05) between samples are indicated by different letters above columns. S175 mutants showed activity similar to that of the wild-type (WT) strain, while the T331A strain had significantly reduced activity and the remaining mutants had no detectable activity. (B) Proteins from the same normalized crude extracts used for the kinase assay were separated via SDS-PAGE (4 to 20%), hybridized to a PVDF membrane, and then probed with anti-GFP primary antibodies to detect PkaC1 isoforms and anti-α-tubulin antibodies as an internal control in Western blot assays. Full-length PkaC1 protein of approximately 80 kDa was detected for WT, S175A, S175E, and T331A samples, but no protein was detected in any of the other samples. The higher-molecular-mass band likely represents an alternate phosphorylation state. (C) Crude extracts from WT and strains overexpressing PkaC1 with native (WT) sequence or the T331E, T333A, and T337E mutations via the constitutive otef promoter were probed in a Western blot assay as described above (upper panel) and also tested for PKA activity as described above (lower panel). While full-length protein was detected in all samples, kinase activity was detected only in samples expressing WT protein.