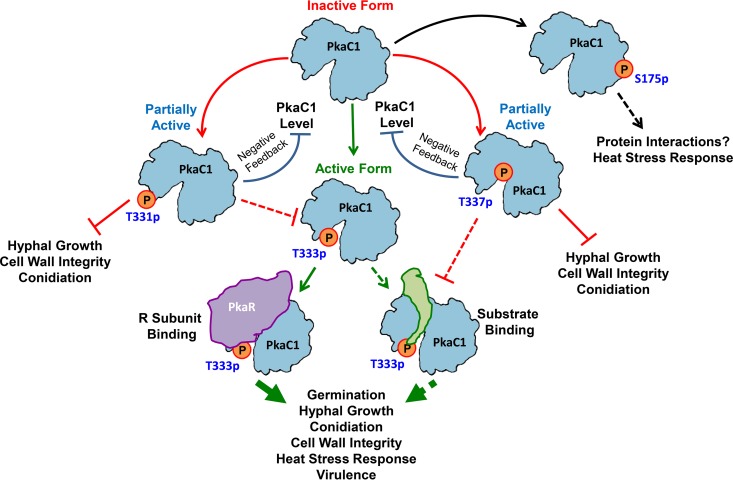
FIG 6 .
Hypothetical model depicting the effect of phosphorylation of PkaC1 at different residues on its activity and function in A. fumigatus. Phosphorylation at T333 leads to activation of PkaC1 via stabilization of substrate and regulatory subunit binding, promoting hyphal growth, conidiation and germination, cell wall integrity, heat stress tolerance, and virulence. In contrast, phosphorylation at T331 or T337 destabilizes substrate binding, causing partial inactivation of PkaC1. In the case of T337 phosphorylation, this likely occurs via blockage of T333 phosphorylation, while T337 phosphorylation is expected to directly inhibit substrate binding due to steric hindrance. As a result, T331 and T337 phosphorylation both lead to inhibition of hyphal growth, conidiation, and cell wall integrity. This suppression of PKA activity also leads to downregulation of PkaC1 protein levels in a negative-feedback mechanism. A dynamic phosphorylation state at S175 is necessary for mounting a normal heat stress response, possibly due to a role for this site in particular unknown protein-protein interactions. Solid lines represent experimentally validated findings, while dashed lines represent hypothesized processes.