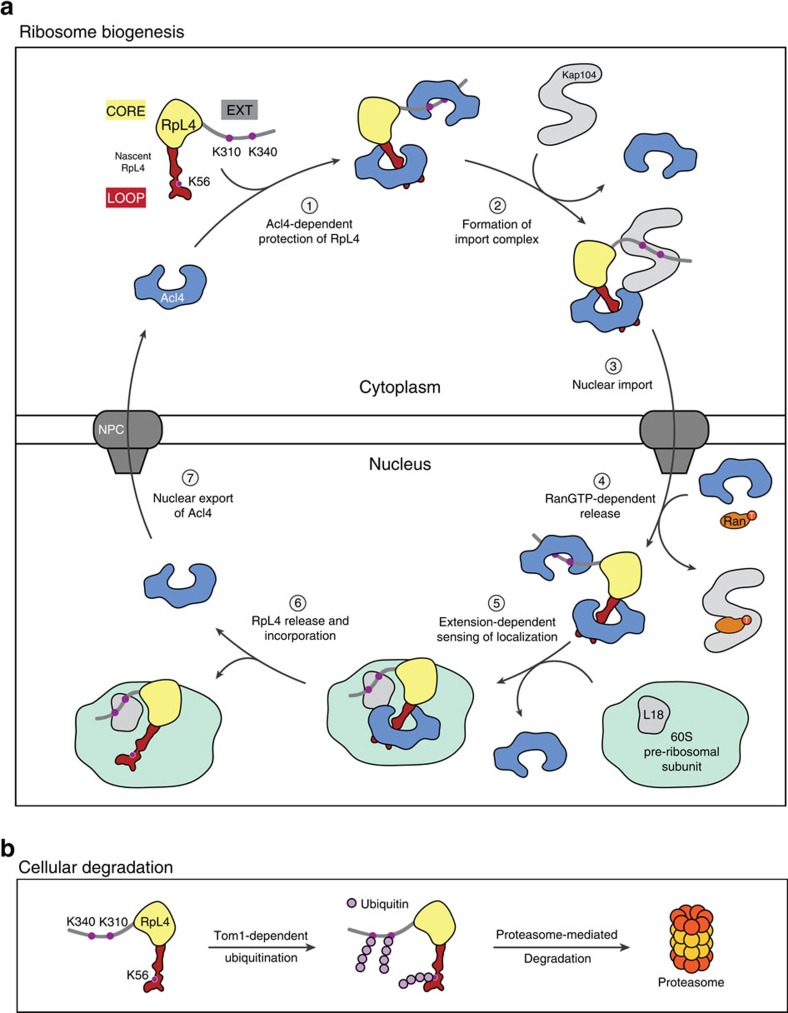
Figure 5. Model for nuclear import and balancing of RpL4.
(a) Acl4- and Kap104-mediated nuclear import of RpL4. The cycle involves seven steps. (1) Following translation, nascent RpL4 is protected by two copies of Acl4 at its unstructured loop and at the unstructured C-terminal extension. (2) A stoichiometric hetero-trimeric nuclear import complex is formed by binding of Acl4·RpL4 to the transport factor Kap104. Kap104 binding occurs in a bi-partite fashion and involves the basic unstructured N-terminal region of Acl4 and RpL4EXT, displacing the RpL4EXT-bound Acl4 copy. (3) Kap104 dependent transport of Acl4·RpL4 through the NPC. (4) After successful transport, the Acl4·RpL4·Kap104 import complex is disassembled by nuclear RanGTP, releasing Acl4·RpL4 into the nucleoplasm. (5) RpL4EXT contacts RpL18 and expansion segment 7 on the surface of the pre-60S subunit10. (6) Constructive interactions result in disassembly of the Acl4·RpL4 complex and incorporation of RpL4 into the large pre-ribosomal subunit. (7) Potential nuclear export of Acl4 allows its entering into the next RpL4 transport cycle. (b) Balancing of excess unassembled ribosomal proteins. In the absence of Acl4 and Kap104, unassembled RpL4 is ubiquitinated by Tom1 and degraded by the proteasome-dependent degradation machinery19.