Abstract
Vanishing bone disease is an extremely rare disorder of unknown etiology characterized by idiopathic osteolysis of bone. We describe a case of vanishing bone disease of chest wall and spine with kyphoscoliosis and neurological deficit. A 17-year-old male presented with gradually progressive deformity of back and dorsal compressive myelopathy with nonambulatory power in lower limbs. Radiographs revealed absent 4th–7th ribs on the right side with dorsal kyphoscoliosis and severe canal narrowing at the apex. The patient was given localized radiotherapy and started on a monthly infusion of 4 mg zoledronic acid. Posterior instrumented fusion with anterior reconstruction via posterolateral approach was performed. The patient had a complete neurological recovery at 5 weeks following surgery. At 1 year, anterior nonunion was noted for which transthoracic tricortical bone grafting was done. Bone graft from the patient's mother was used both times. At 7 months following anterior grafting, the alignment was maintained and the patient was asymptomatic; however, fusion at graft-host interface was not achieved. Bisphosphonates and radiotherapy were successful in halting the progress of osteolysis.
Keywords: Chest wall, Gorham disease, idiopathic osteolysis, kyphosis, vanishing bone disease
MeSH terms: Osteolysis, spine. chest wall, kyphosis, grafting, bone
INTRODUCTION
Vanishing bone disease, popularly known as Gorham's disease (GD), is an extremely rare disorder of unknown etiology. The pathogenesis and treatment of this disorder is still a mystery. Though known as Gorham–Stout disease, it was first described by Jackson in 1838.1 In 1955, Gorham and Stout described a series of 24 patients giving the first detailed description of this disorder and hence it got the name.2 Since then, it has been known as disappearing bone disease, acute bone resorption, phantom bone disease, vanishing bone disease, and idiopathic massive osteolysis.3
The hallmark of this disease is osteolysis of the involved bone in the absence of infective/malignant or immunological etiology. GD is a diagnosis of exclusion. The criteria for diagnosis suggested by Heffez et al. and modified by Choma et al. are shown in Table 1.4,5 Nonoperative treatment options include bisphosphonates, radiotherapy, and various drugs used alone or in combination. Operative reconstruction of the affected bone has high failure rates due to resorption of the bone graft used.6 We describe the nonoperative and operative management of a patient with GD involving chest wall and spine, who presented with kyphoscoliosis and neurological deficit.
Table 1.
Heffez criteria for diagnosis of Gorham's disease (modified by Choma)

CASE REPORT
A 17-year-old male presented with history of gradually progressive deformity in the back since 3 months and weakness in both lower limbs since 3 days. He had no history of trauma, fever, or similar complaints in the family. There was no significant medical/surgical history. On examination, he had dorsal scoliosis with convexity to the right with angular proximal dorsal kyphosis. His chest was flattened on the right side and the 4th–7th ribs on the right side were absent on palpation. He had non-ambulatory power in his lower limbs (power around the hip and knee was 2/5 and power around ankle and in toes was 3/5) with spasticity and exaggerated deep tendon reflexes. Hypoesthesia below D7 was noted and bowel bladder sensation was preserved. Plantar response was extensor.
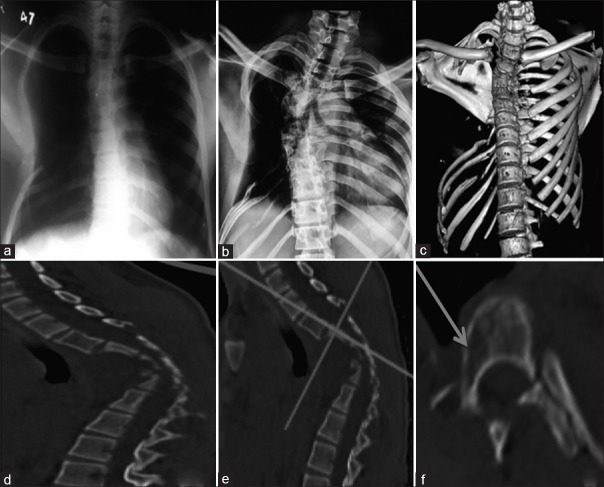
An X-ray taken elsewhere 6 months prior to presentation to OPD showed mild scoliosis with few ribs absent on the right side [Figure 1a]. Due to suboptimal quality of the X-ray, we could count which ribs were absent. X-ray taken in our OPD demonstrated dorsal scoliosis with convexity to the right side, apex at D6, and Cobbs angle of 41° with angular dorsal kyphosis with apex at D5–D6. The 4th to 7th ribs on the right side were absent with destruction in the lower ribs more as compared to the X-ray taken 6 months back [Figure 1b].
Figure 1.
(a) Chest X-ray taken 6 months prior to presentation to us showing mild scoliosis and few absent ribs on the right side (b) X-ray at presentation to us showed right-sided scoliosis with Cobb's angle 41°. Destruction of ribs has increased as compared to Figure 1a. (c) Three-dimensional reconstruction of the chest (Sternum has been digitally subtracted for better visualization of chest wall.) showing absent ribs on (R) side (4th to 7th rib) (d) Sagittal view showing severe narrowing of the canal at the apex of kyphosis. (e) Dicom image showing the level at which image in Figure 1f has been taken. (f) Axial cut showing osteolysis (arrow) in body and pedicle of vertebrae proximal to the apex
Computed tomography (CT) scan further illustrated the pathoanatomy of the disorder. All X-ray findings were confirmed. Three-dimensional reconstruction showed a complete absence of the right 4th–7th ribs [Figure 1c]. There was a severe narrowing of the canal at the apex. In addition, right-sided pedicles and right half of vertebral bodies proximal to the apex showed osteolysis [Figure 1e and f]. There was severe narrowing of the canal at the apex of kyphosis [Figure 1d]. Magnetic resonance imaging (MRI) showed stretching and compression of the cord at the apex of the deformity. There was no evidence of abscess/inflammation. Angiography did not reveal any abnormality.
The patient was given zoledronic acid infusion (4 mg) every month. Zoledronic acid was stopped at 18 months and patient was switched to weekly alendronate with a dose of 70 mg per week. He was also given radiotherapy at a dose of 8 Gy (single dose) 2 weeks prior to surgery. This dose was given after consultation with a senior radiotherapy consultant at a tertiary care hospital specializing in cancer treatment.
The patient was taken for surgery in prone position under general anesthesia. Following midline exposure, bilateral T8–T12 pedicle screws were passed. Pedicle screws were passed on the left side from T1 to T3. The right-sided pedicle and body were cystic as confirmed by palpating with a ballpoint probe. Hence, MRI-compatible sublaminar cables were passed from T1 to T3 on the right side. D5 and D6 laminae were soft and were excised. Thorough cord decompression was done. D4 and D5 bodies were excised. The excised bones were sent for histopathological examination. Contoured rod was applied and sequentially tightened. The deformity was fully corrected following fixation. The proximal end of the right-sided rod was bent and the bent part was put between the spinous process of C7 and T1 to provide additional stability.
Bilateral fibulae and cancellous graft from right posterior iliac crest were harvested from the patient's mother after both patient and mother were found to be ABO and Rh compatible in preoperative blood testing. The mother's bone was used as there were chances that the harvested patients bone might get resorbed. Anterior reconstruction was done via posterolateral approach using cancellous bone graft and two pieces of strut fibulae. Posterior fusion was done using two long struts of fibula bridging the laminectomy defect. Additional matchstick graft prepared from remaining fibulae was placed posteriorly [Figure 2c]. The wound was closed over a drain.
Figure 2.
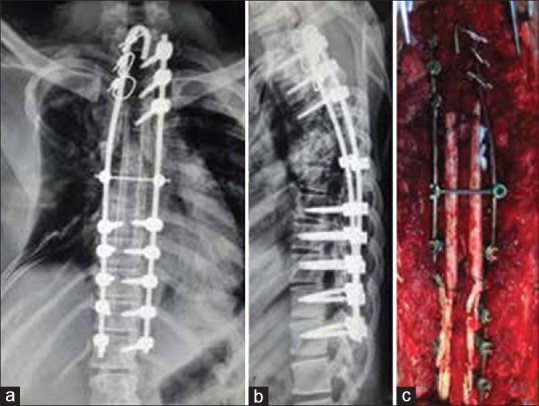
(a and b) Postoperative X-ray dorsal spine anteroposterior and lateral views showing full correction of scoliosis with implant in situ (c) Peroperative photograph showing posterior fixation and reconstruction using fibulae
Histopathology revealed decreased bony trabeculae with irregular vascular formations. The bony trabeculae seemed to be replaced by fibrous tissue. The fibrous tissue demonstrated not only increased number of blood vessels but also the absence of cellular atypia/granulomas. The number of osteoclastic giant cells were increased. The material was negative for any fungal or bacterial organisms. No specific diagnosis was obtained on histology. Due to the unavailability of facility to take microscopic images at our institute, the pathology images could not be provided.
Postoperative X-ray showed full correction of deformity [Figure 2a and b]. The drain was removed on 4th postoperative day. The patient started regaining power on 7th postoperative day and had a complete neurological recovery at 5-week postoperatively. The patient was mobilized with a total contact brace. X-rays at followup showed that resorption of ribs had not progressed. X-ray at 1-year postoperatively showed that alignment is maintained [Figure 3a and b]; however, consolidation of the anterior graft was not seen. A CT scan showed an anterior void and the fibulae had not united with the host bone [Figure 3c].
Figure 3.
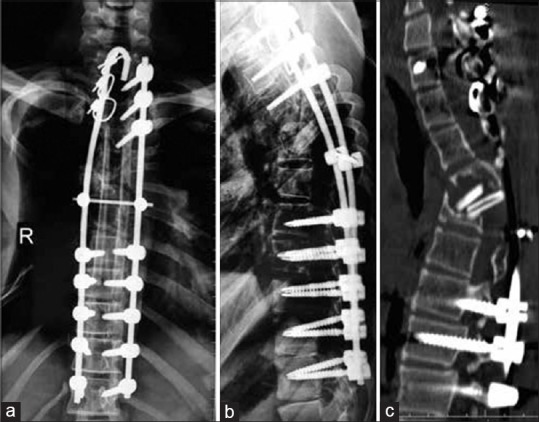
(a and b) Radiographs of dorsal spine anteroposterior and lateral views at 1 year followup showing maintenance of alignment; however, anterior fusion is not seen. (c) Computed tomography scan at one year showing anterior void and fibulae had not united
The patient was taken for surgery in the lateral position and the nonunion site exposed via left transthoracic approach. No rib was excised and the plane between the 5th and 6th rib was utilized to reach the nonunion site. Both fibulae struts were removed and fibrous tissue excised. The mother's tricortical iliac crest bone graft was harvested and used for anterior reconstruction.
At 7-month followup, the patient has no neurological deficit and walks with a total contact brace. Radiographs revealed maintained alignment [Figure 4a and b]. CT showed that anterior strut graft has not resorbed; however, it still has not united with the host bone [Figure 4c]. Since patient remained asymptomatic, we have kept the patient on monthly monitoring with X-ray check.
Figure 4.
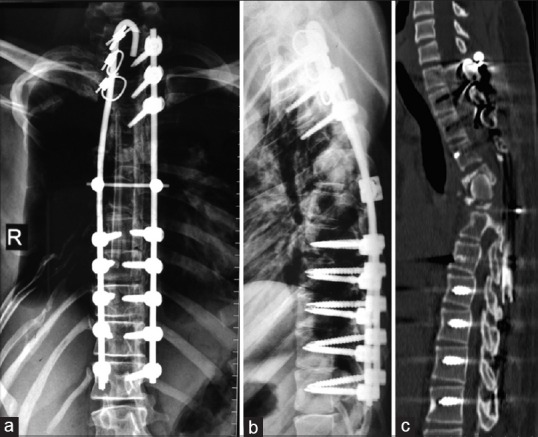
(a and b) Anteroposterior and lateral radiographs of dorsal spine at 7 months followup showing maintenance of alignment. (c) Computed tomography scan at 7 months following anterior reconstruction showing that tricortical graft has not resorbed but no fusion at graft host bone interface
DISCUSSION
The mechanism of bone resorption in GD is still unclear. Increased activation of local osteoclasts is the foremost theory. Heyden et al. noticed that perivascular cells in areas of osteolysis might act as osteoclast precursors as they demonstrate strong acid phosphatase and leucine aminopeptidase activities.7 This leads to an increase in local osteoclastic activity causing resorption of bone. Histologic studies have shown increased proliferation of vascular and lymphatic tissues in areas of osteolysis. Although the exact mechanism of this proliferation is not known, cytokines involved in angiogenic pathways such as platelet-derived growth factor, interleukin 6 (IL-6), vascular endothelial growth factor (VEGF), and lymphatic vessel endothelial hyaluronan receptor 1 may be involved.8 Mechanical pressure caused by increased vascular tissue may be one of the causes of resorption of bone. Other mechanisms proposed are dysplasia of local lymphatic and blood vessels, primary aberration of vascular tissue due to granulation tissue in bone, and activation of local hydrolytic enzymes due to local hypoxia and acidosis induced by angiomatosis.3,9,10
Four stages have been described for the radiologic appearance of GD.3 The first stage involves the appearance of intramedullary and subcortical radiolucent foci resembling patchy osteoporosis. Concentric shrinkage in tubular bones with tapering of the involved ends occurs in the second stage. This results in a “sucked candy” appearance. In the third phase, cortical erosion along with local invasion of the angiomatous mass into the adjacent soft tissue appears. The fourth stage is characterized by the disappearance of the remaining bone. Progression of the osteolytic process into multiple contiguous bones may occur at this stage.
Gorham and Stout described a marked increase in the number of capillaries first within the affected bones, which are found to be dilated and filled with red blood cells.2 They noticed that these vessels do not disappear after bone resorption but remain as a vascular growth closely resembling that found within the marrow spaces, or as a sponge-like meshwork of anastomosing vascular spaces. Histological examination of the affected bone shows replacement of absorbed bone by fibrous connective tissue, with marked proliferation of thin-walled vessels and anastomosing dilated endothelium-lined spaces that sometimes contain red blood cells. King suggested “hemangiomatosis” for description of these pathological findings.11
Multiple reports have shown the efficacy of bisphosphonates in the treatment of GD.6,9,12,13 Some histologic studies have suggested that osteolysis in GD is due to increased local number of stimulated osteoclasts. By inhibiting the action of osteoclasts, bisphosphonates prevent resorption of bone. In addition to this, bisphosphonates may normalize serum levels of IL-6, thus reducing the osteoclastic activity.8 Bisphosphonates have also shown to be having good analgesic effect. Bisphosphonates have not shown to cause bone formation in areas of osteolysis, but they have demonstrated efficacy in prevention of further resorption of bone and reduction of local symptoms. However, the dosage and duration for which bisphosphonates are to be used is uncertain. A case report showed the use of bisphosphonates for a period of 17 years with moderate success.12 Another report showed the efficacy of monthly dose of 4 mg of zoledronic acid given for a period of 12 months.14 We used 4 mg zoledronic acid infusion every month for 18 months while closely monitoring for any side effects. Usually, zoledronic acid is given at a dose of 4 mg once a year. The long term effects of monthly infusion of zoledronic acid are not known as such high dose has been rarely used. There have been previous reports which have shown the efficacy of oral bisphosphonates in the treatment of GD. In addition, indoor admission is needed for giving IV infusion and due to financial constraints, our patient could not afford monthly infusions of zoledronic acid. Due to these reasons, after 18 months the patient was started on oral alendronate at a dose of 70 mg weekly.
Localized radiotherapy has also shown to be effective in halting the progression of disease with a few reports also showing new bone formation.3,6,15 The exact mechanism is not known but it is postulated that radiotherapy may arrest endothelial cell proliferation and limit the progression of disease.16,17 Radiation doses of 40–45 Gy in 2 Gy fractions have been shown to yield remission rates as high as 60%. Radiotherapy may also be used in patients who are poor surgical candidates and also patients who have failed surgical treatments. Recalcification which is exceedingly rare with other treatment modalities was shown by Choma et al. in 5 of 18 cases of GD treated with radiotherapy.5 Following the use of bisphosphonates and radiotherapy, we noted a halt in the progression of osteolysis [Figure 5].
Figure 5.
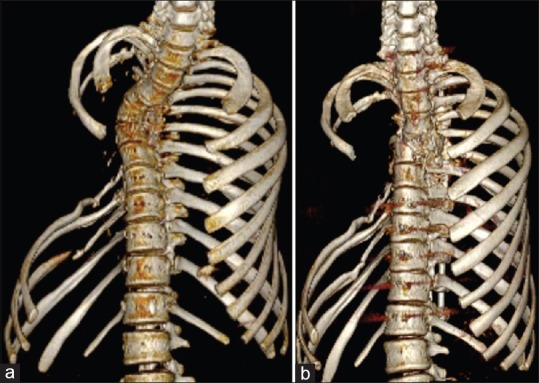
Three-dimensional reconstruction of chest wall before (a) and 18 months after (b) radiotherapy and monthly zoledronic acid (scapula and sternum digitally subtracted) showing that osteolysis in the ribs has not progressed
Among other medical therapies, interferon alpha-2b has been used.17,18 The proposed mechanism of action is down-regulation of VEGF, which leads to the inhibition of vascular proliferation. It may also inhibit proliferation and migration of lymphatic endothelial cells. It suppresses the growth of both osseous and soft tissue lesions. If used for treatment, long term use has to be done (usually more than 1 year) as symptoms tend to improve after 10–16 months. Moreover, it has many side effects like hematological toxicity, hepatotoxicity, nausea, and psychiatric side effects. Thus, it has to be used with caution and strict monitoring. Bevacizumab is another VEGF inhibitor which has been successfully used in one pediatric patient with GD.19 There is no current evidence for its use for GD in adults.
GD of spine is extremely rare. Aizawa et al. published a review of 28 cases of GD of spine in 2005.6 To the best of our knowledge, 13 cases of GD of spine have been reported after 2005.8,20,21,22,23,24,25,26,27,28,29,30,31 A brief review of these cases with treatment and outcome has been shown in Table 2. Out of the total 41 reported cases of GD of spine, cervical spine is the most commonly affected with 11 cases involving only cervical spine and additional 6 cases involving cervicothoracic spine. Thoracic spine is the second most commonly affected area. Majority of the patients with spine involvement are less than 20 years old. GD of spine is more common in males (n = 28) as compared to females (n = 13).
Table 2.
Review of cases of Gorham disease of spine reported in the last 10 years
Pleural effusion has been reported in 22 of the 41 cases of GD of spine. Of these, 9 patients expired (mortality of 41%). Most of these effusions were due to chylothorax. Chylothorax is thought to be due to disruption of thoracic duct or pleural lymphatics by adjoining osteolysis. Due to high mortality, any evidence of pleural effusion or respiratory discomfort must not be ignored and investigated promptly.
Out of the 41 cases reported, surgical management was done in 18 patients. Neurological deficit is the most common indication for surgery. Posterior instrumented fusion is the most common surgery performed; however, the rates of fusion are very poor. Only 4 of the patients have documented fusion following the first surgery and fusion was not accomplished in nearly half of the patients even after multiple surgeries.6 Use of bone morphogenetic protein-2 (BMP-2) may enhance fusion rates.8 Due to financial constraints, we could not use allograft or BMP-2 in our patient. Autografts have high rates of resorption when used for reconstruction. Hence, his mother's bone was utilized for reconstruction after ABO and Rh compatibility testing. Two long pieces of fibula from the mother were placed posteriorly to bridge the affected area. The grafted bone did not get resorbed but probably due to the pathological bone at graft-host interface, the anterior union could not be achieved following the first surgery. Posterior fusion could not be assessed on CT scan due to interference from the implant. A second transthoracic anterior grafting using mother's tricortical iliac crest graft was done. CT scan at 7 months still showed that although the graft did not get resorbed, the union at the graft-host interface was not present.
After managing this case, we learned that in the presence of neurological deficit, decompressive surgery must be done even though fusion rates are poor. Adequate decompression and deformity correction gives excellent neurological recovery. Bisphosphonates and localized radiotherapy are effective in halting the progression of osteolysis. A regular followup and CT scan are necessary to diagnose nonunion at the earliest. If left untreated, nonunions are known to cause implant failure, hence revision surgery and bone grafting using strut graft must be done if required.
Vanishing bone disease is extremely rare and its etiology and pathogenesis is still not clear. Bisphosphonates and local radiotherapy are effective in halting the progress of osteolysis. Since pedicles and body might be cystic, one must be prepared to use sublaminar wires for fixation. Achieving union in such patients is difficult and multiple surgeries may be needed to achieve union.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jackson JB. A boneless arm. Boston Med Surf. 1838;18:368–9. [Google Scholar]
- 2.Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37-A:985–1004. [PubMed] [Google Scholar]
- 3.Daneshvar Kakhaki A, Khodadad K, Pejhan S, Karimi S, Arab M, Saghebi R, et al. Gorham's disease with chest wall involvement: A case report and a review of the literature. Iran Red Crescent Med J. 2014;16:e12180. doi: 10.5812/ircmj.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55:331–43. doi: 10.1016/0030-4220(83)90185-8. [DOI] [PubMed] [Google Scholar]
- 5.Choma ND, Biscotti CV, Bauer TW, Mehta AC, Licata AA. Gorham's syndrome: A case report and review of the literature. Am J Med. 1987;83:1151–6. doi: 10.1016/0002-9343(87)90959-4. [DOI] [PubMed] [Google Scholar]
- 6.Aizawa T, Sato T, Kokubun S. Gorham disease of the spine: A case report and treatment strategies for this enigmatic bone disease. Tohoku J Exp Med. 2005;205:187–96. doi: 10.1620/tjem.205.187. [DOI] [PubMed] [Google Scholar]
- 7.Heyden G, Kindblom LG, Nielsen JM. Disappearing bone disease. A clinical and histological study. J Bone Joint Surg Am. 1977;59:57–61. [PubMed] [Google Scholar]
- 8.Maillot C, Cloche T, Le Huec JC. Thoracic osteotomy for Gorham-Stout disease of the spine: A case report and literature review. Eur Spine J. 2014 doi: 10.1007/s00586-014-3613-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Young JW, Galbraith M, Cunningham J, Roof BS, Vujic I, Gobien RP, et al. Progressive vertebral collapse in diffuse angiomatosis. Metab Bone Dis Relat Res 1983. 1984;5:53–60. doi: 10.1016/0221-8747(83)90001-2. [DOI] [PubMed] [Google Scholar]
- 10.Thompson JS, Schurman DJ. Massive osteolysis. Case report and review of literature. Clin Orthop Relat Res. 1974;103:206–11. [PubMed] [Google Scholar]
- 11.King DJ. A case resembling hemangiomatosis of the lower extremity. J Bone Joint Surg Am. 1946;28:623–8. [PubMed] [Google Scholar]
- 12.Lehmann G, Pfeil A, Böttcher J, Kaiser WA, Füller J, Hein G, et al. Benefit of a 17-year long term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129:967–72. doi: 10.1007/s00402-008-0742-3. [DOI] [PubMed] [Google Scholar]
- 13.Patel DV. Gorham's disease or massive osteolysis. Clin Med Res. 2005;3:65–74. doi: 10.3121/cmr.3.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, Andrade ES. Use of zoledronic acid in the treatment of Gorham's disease. Int J Pediatr Otorhinolaryngol. 2010;74:319–22. doi: 10.1016/j.ijporl.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Hu P, Yuan XG, Hu XY, Shen FR, Wang JA. Gorham-Stout syndrome in mainland China: A case series of 67 patients and review of the literature. J Zhejiang Univ Sci B. 2013;14:729–35. doi: 10.1631/jzus.B1200308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyd R, Micke O, Surholt C, Berger B, Martini C, Füller J, et al. Radiation therapy for Gorham-Stout syndrome: Results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81:e179–85. doi: 10.1016/j.ijrobp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Dunbar SF, Rosenberg A, Mankin H, Rosenthal D, Suit HD. Gorham's massive osteolysis: The role of radiation therapy and a review of the literature. Int J Radiat Oncol Biol Phys. 1993;26:491–7. doi: 10.1016/0360-3016(93)90968-2. [DOI] [PubMed] [Google Scholar]
- 18.Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis- Gorham-Stout syndrome: A marker of activity? A case report with a 5-year followup. Bone. 2010;46:873–6. doi: 10.1016/j.bone.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Grunewald TG, Damke L, Maschan M, Petrova U, Surianinova O, Esipenko A, et al. First report of effective and feasible treatment of multifocal lymphangiomatosis (Gorham-Stout) with bevacizumab in a child. Ann Oncol. 2010;21:1733–4. doi: 10.1093/annonc/mdq331. [DOI] [PubMed] [Google Scholar]
- 20.Adler F, Gupta N, Hess CP, Dowd CF, Dillon WP. Intraosseous CSF fistula in a patient with Gorham disease resulting in intracranial hypotension. AJNR Am J Neuroradiol. 2011;32:E198–200. doi: 10.3174/ajnr.A2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahoo RK, Jagannathan B, Palanichamy G, Natarajan V. Anaesthetic consideration in patients with Gorham's syndrome: A case report and review of the literature. Indian J Anaesth. 2012;56:391–3. doi: 10.4103/0019-5049.100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekharappa V, Arockiaraj J, Amritanand R, Krishnan V, David KS, David SG. Gorham's disease of spine. Asian Spine J. 2013;7:242–7. doi: 10.4184/asj.2013.7.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esmailiejah AA, Kamalian N, Abbasian M. Temporary paraplegia resulting from Gorham's disease involving the third lumbar vertebra and proximal femur: A five-year followup and review of the literature. Arch Iran Med. 2013;16:686–90. [PubMed] [Google Scholar]
- 24.Barman A, Bhide R, Viswanathan A, George J, Thomas R, Tharion G. Gorham's disease of the spine. NeuroRehabilitation. 2013;33:121–6. doi: 10.3233/NRE-130935. [DOI] [PubMed] [Google Scholar]
- 25.Huang SY, Lee YM, Tzeng ST, Su CP, Huang SF, Wu YK, et al. Gorham syndrome with postoperative respiratory failure and requiring prolonged mechanical ventilation. Respir Care. 2013;58:e144–8. doi: 10.4187/respcare.02355. [DOI] [PubMed] [Google Scholar]
- 26.Lala S, Mulliken JB, Alomari AI, Fishman SJ, Kozakewich HP, Chaudry G. Gorham-Stout disease and generalized lymphatic anomaly – Clinical, radiologic, and histologic differentiation. Skeletal Radiol. 2013;42:917–24. doi: 10.1007/s00256-012-1565-4. [DOI] [PubMed] [Google Scholar]
- 27.Kilicoglu ZG, Kizildemir Kis N, Vardar Aker F, Berkman MZ, Simsek MM. Gorham disease of the craniocervical junction: X-ray, computed tomography, and magnetic resonance imaging findings. Spine J. 2013;13:e11–4. doi: 10.1016/j.spinee.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 28.Suero Molina EJ, Niederstadt T, Ruland V, Kayser G, Stummer W, Ewelt C, et al. Cerebrospinal fluid leakage in Gorham-Stout disease due to dura mater involvement after progression of an osteolytic lesion in the thoracic spine. J Neurosurg Spine. 2014;21:956–60. doi: 10.3171/2014.8.SPINE131064. [DOI] [PubMed] [Google Scholar]
- 29.Kakuta Y, Iizuka H, Kobayashi R, Iizuka Y, Takahashi T, Mohara J, et al. Gorham disease of the lumbar spine with an abdominal aortic aneurysm: A case report. Spine J. 2014;14:e5–9. doi: 10.1016/j.spinee.2013.07.451. [DOI] [PubMed] [Google Scholar]
- 30.Carbó E, Riquelme Ó, García A, González JL. Vertebroplasty in a 10-year-old boy with Gorham-Stout syndrome. Eur Spine J. 2015;24(Suppl 4):S590–3. doi: 10.1007/s00586-015-3764-x. [DOI] [PubMed] [Google Scholar]
- 31.Kim MK, Hong JR, Kim SG, Lee SK. Fatal progression of Gorham disease: A case report and review of the literature. J Oral Maxillofac Surg. 2015 doi: 10.1016/j.joms.2015.06.154. pii: S0278-239100822-8. [DOI] [PubMed] [Google Scholar]