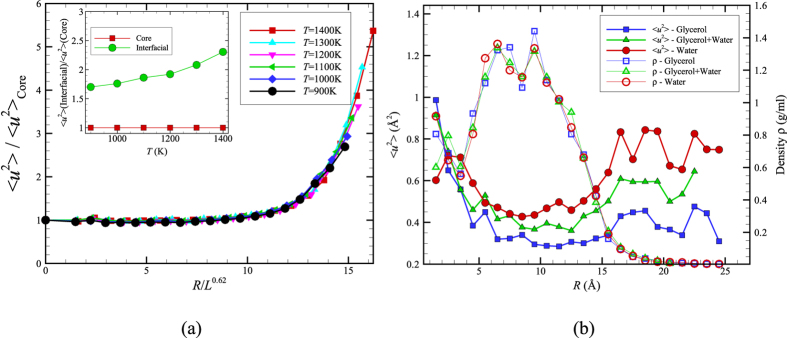
Figure 2. Radially averaged Debye-Waller factor 〈u2〉 for a Ni NP versus the atoms of ubiquitin.
(a) The 〈u2〉 values for the Ni NP atoms with R = 2 nm (whose interatomic interactions are modeled by the Voter-Chen Embedded Atom Method (EAM) potential53) are normalized by their value at the center of the NP and R is the radial distance from the NP center13. Inset compares 〈u2〉 values in the NP interfacial region to values in the NP core. All our radially averaged 〈u2〉 data can be described as universal function of R/L0.62 where L is the string length (defined in text). The width of the interfacial region of enhanced mobility near the NP surface is apparently governed by the scale of collective exchange motion within the NP13. Recent work has demonstrated that L also governs the width of both the polymer-air interfacial layer of a glass-forming polymeric liquid film34 and the interfacial layer of bulk crystalline Ni14 so that the existence of a mobile interfacial near the ‘softening temperature’ of the material seems to be a general property of condensed materials broadly. (b) The radial averaged 〈u2〉 of ubiquitin atoms in glycerol, water, and a glycerol-water mixture at T = 300 K where R is the radial distance from the protein center of mass. The addition of TIP3P water to the glycerol solution clearly ‘plasticizes’ the ubiquitin dynamics, i.e., increases the amplitude of local atomic displacements within the protein where this effect is greater near the protein periphery than the protein core. We also show the radially averaged local density ρ within the protein to emphasize that the local density ρ and the local ‘mobility’ 〈u2〉 do not exhibit similar trends with R in this protein. We show in Fig. 3 that this is not a universal behavior in the dynamics of proteins.