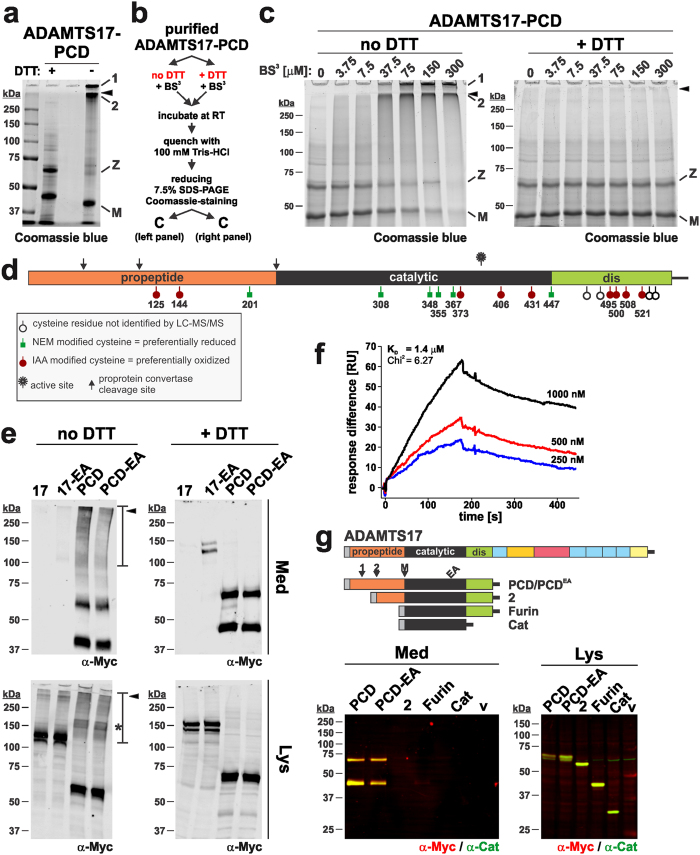
Figure 4. ADAMTS17-PCD self-associates via disulfide bonds involving the propeptide.
(a) Coomassie blue stained 7.5% SDS-PAGE of purified ADAMTS17-PCD (20 μg/lane) separated under reducing (+ DTT) and non-reducing (− DTT) conditions. The black arrowhead depicts the border between the stacking and separating gel; 1 and 2 indicate large molecular weight complexes of ADAMTS17-PCD, which in the absence of DTT, did not enter the stacking or separating gel, respectively. The “+ DTT” and “− DTT” lanes are separated by an empty lane. M, mature enzyme; Z, zymogen. (b) Flow chart outlining the design of cross-linking experiments in c. (c) Coomassie blue-stained reducing SDS-PAGE of purified ADAMTS17-PCD (10 μg/lane) incubated with increasing concentrations of BS3 cross-linker in the presence (+ DTT) or absence of reducing agent (no DTT) (Annotations as in a). (d) Schematic of ADAMTS17-PCD showing cysteine residues and indicating their oxidized or reduced status determined by LC-MS/MS. (e) Western-blot analysis of conditioned medium (Med) and cell lysate (Lys) from cells expressing ADAMTS17 (17), ADAMTS17EA (17-EA), ADAMTS17-PCD (PCD), and ADAMTS17-PCDEA (PCD-EA) under reducing (+ DTT) and non-reducing conditions (no DTT). Areas of the gel in the high MW range (>100 kDa) reacting with anti-myc in the absence of DTT in medium and lysate are outlined with a bar. The asterisk indicates prominent anti-myc reactive ADAMTS17-PCD band in the lysate under non-reducing conditions. (f) Surface plasmon resonance indicates interaction of ADAMTS17-PCD to surface immobilized ADAMTS17-PCD in a dose dependent manner. The dissociation constant (KD) was calculated assuming 1:1 binding. (g) Schematic of ADAMTS17-PCD truncation mutants (top panel). Western blot analysis of conditioned medium and cell lysate from HEK293 cells transiently transfected with ADAMTS17-PCD truncation mutants indicates anti-myc and anti-catalytic domain antibody reactive bands (yellow) of the predicted molecular weight in cell lysates for each mutant (bottom, right-hand panel). However, the entire propeptide is required for secretion (bottom, left-hand panel).