Abstract
Background
The abnormal activity of Sirtuin 1 (Sirt1) is closely related to the aging of vascular endothelial cells. As a bioactive molecule, allicin has antioxidant, anti-inflammatory, and lipid-regulating mechanisms. However, few reports about the relationship of allicin and Sirt1 have been published. In this study, we aimed to elucidate the effect of allicin on Human Umbilical Vein Endothelial Cells (HUVECs) aging induced by hydrogen peroxide (H2O2) and the role of Sirt1 in this phenomenon.
Material/Methods
HUVEC were exposed to H2O2 to establish the aging model. The expression of protein and RNA were detected by Western blot and Reverse transcription-quantitative polymerase chain reaction. The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess cell viability. Sirt1 enzyme activity assay was used to analyze enzymatic activity. Reactive oxygen species was detected by dichlorofluorescein diacetate (DCFH-DA). Cell aging was detected by Senescence β-Galactosidase (SA-β-gal) staining.
Results
Results of this study revealed that pretreating HUVECs with 5 ng/mL allicin before exposure to H2O2 resulted in increased cell viability and reduced reactive oxygen species generation. Western blot and quantitative real-time polymerase chain reaction (qRT-PCR) analysis showed that H2O2 attenuated the phosphorylation and activation of Sirt1 and increased the expression of plasminogen activator inhibitor-1(PAI-1) protein. Moreover, H2O2 also promoted HUVEC aging. These effects were significantly alleviated by 5 ng/mL allicin co-treatment. Furthermore, the anti-aging effects of allicin were abolished by the Sirt1 inhibitor nicotinamide (NAM).
Conclusions
Overall, the results demonstrated that allicin protects HUVECs from H2O2-induced oxidative stress and aging via the activation of Sirt1.
MeSH Keywords: Aging, Atherosclerosis, Biological Factors, Cardiovascular Agents
Background
Atherosclerosis is one of the main causes of cardiovascular diseases; thus, atherosclerosis prevention is essential for cardiovascular disease treatment. Endothelial cell injuries initiate atherosclerosis occurrence, and endothelial cell aging is one of the main causes of endothelial cell injuries. Numerous studies have shown that endothelial cell aging and dysfunction are the key factors leading to cardiovascular injuries, and endothelial cell aging is closely related to atherosclerosis [1,2]. Thus, the reverse aging of endothelial cells is significant in atherosclerosis prevention.
Allicin is a biologically active compound extracted from garlic; it has anti-inflammatory, antioxidant, and lipid-regulating effects [3,4]. Li et al. [5] found that allicin could inhibit the occurrence and development of atherosclerosis and reduce plasma lipid concentrations in apoE−/− and low-density lipoprotein receptor double knock-out (LDLR−/−) mice on a high-fat diet. Results of the aforementioned studies show that allicin has anti-atherosclerosis effects. Reactive oxygen species (ROS) is a contributing factor to cell damage and a cause of cell aging, as demonstrated in a previous study [6]. Liu et al. [7] conducted in vivo experiments and found that allicin could prevent endothelial damage induced by lipid peroxidation. Chan et al. [8] likewise confirmed that allicin protected cardiac cells against hydrogen peroxide. However, the role of allicin in the reverse aging of endothelial cells has not yet been reported.
Sirt1 is a deacetylase dependent on nicotinamide-adenine dinucleotide and is a member of the mammalian SIRT family. Numerous studies have confirmed that Sirt1 activation has an anti-aging effect. Moreover, several studies have reported that Sirt1 can inhibit apoptosis, regulate metabolism (e.g., calorie intake and fat storage), maintain the normal function of mitochondria under oxidative stress, present anti-inflammatory effect, and inhibit or delay cell aging [9,10]. Atherosclerosis studies have shown that Sirt1 inhibits apoptosis induced by oxidized low-density lipoprotein (ox-LDL) in endothelial cells and reduces smooth muscle hypertrophy induced by angiotensin II (AngII), which has an insulin resistance effect on atherosclerosis [11,12]. In this study, HUVECs were treated with H2O2 to establish an endothelial cell aging model, the effects of allicin on HUVECs aging were observed, and the activation of Sirt1 was detected. Finally, whether Sirt1 is involved in the inhibitory effect of allicin on H2O2-induced HUVEC aging was investigated.
Material and Methods
Materials
HUVECs was purchased from Cell Culture Center, Institute of Biochemistry and Cell Biology, Chinese, Academy of Life Sciences (Shanghai, China); RPMI 1640 medium and fetal bovine serum (FBS) were acquired from HyClone (GE Healthcare Life Sciences, Logan, UT, USA); TRIzol reagent, cDNA synthesis kit was purchased from Invitrogen (Thermo Fisher Scientific, Inc. Waltham, MA, US); allicin was purchased from Sigma-Aldrich (St. Louis, MO, USA); epigenase universal Sirt1 activity assay Kit was purchased from YongBio (catalogue number: GMS50287.2; ShangHai, China); PAI-1, Sirt1, p-Sirt1 were purchased from Santa Cruz Biotechnology Inc., (Santa Cruz, CA, USA); cell lysis buffer was purchased from Beijing ComWin Biotech (Beijing ComWin Biotech, Beijing, China); senescence β-Galactosidase Staining Kit, MTT and Reactive Oxygen Species Assay Kit were obtained from Beyotime (Beyotime, Shanghai, China); and endothelial cell growth supplement (ECGS) was purchased from ScienCell Research Laboratories, Inc (Carlsbad, CA, USA).
Cell Culture
HUVECs were cultured in RPMI-1640 mediums with 10% fetal bovine serum (FBS) and 2% ECGS at 37°C in 5% CO2 atmosphere. HUVECs were passaged every 2 days. HUVECs cells were seeded at 4×106 cells/dish with 10% calf serum incubated for 24 h. In addition, HUVECs were cultured without serum for 6 h before the experiments were started.
MTT assay
The MTT assay was used to assess cell viability. Before each experiment, HUVECs (8000 cells/well) were seeded in 96-well microtiter plates, and treated with 25 μmol/L H2O2 for 1 h to established the aging model, and then HUVECs with allicin (5 ng/mL) and allicin (5 ng/mL) +NAM or NAM for 24 h. Subsequently, 10 μl MTT solution was added to each well, and the plates were incubated for 4 h at 37°C. The absorbance was measured at 470 nm and used to calculate the relative ratio of cell viability.
Measurements of Intracellular ROS
The intracellular ROS level was measuring by a fluorescent product formed through the oxidation of DCFH-DA (Sigma-Aldrich; St Louis, MO, USA). Briefly, HUVECs with allicin (5 ng/mL) and allicin (5 ng/mL)+NAM or NAM for 24 h, then culture medium was removed and washed in PBS 3 times, and then we added fresh culture medium. At the same time, HUVECs were incubated with DCFH-DA (10 umol/L) at 37°C for 30 min. Finally, the relative amount of fluorescent product was assessed using a fluorescence microscope connected to an imaging system (BX50-FLA; Olympus). The median fluorescence intensity (MFI) from 5 random fields was measured using Image 11.41of software and the MFI was used as an index of the amount of ROS.
Enzymatic activity assay
A Sirt1 enzyme activity assay was performed to determine the effect of allicin on activity using a commercially available kit (Genmed, Plymouth, MN, USA). After preparing cell lysates, the Sirt1 activity assay was performed in a 96-well plate according to the manufacturer’s instructions. The reaction product emitted fluorescence, which was detected using an excitation wavelength of 350 nm and an emission wavelength of 405 nm.
Western blot analysis
Cells were lysed in RIPA buffer (catalogue number, P0013; Beyotime, Shanghai, China) and 1 mmol/L phenyl methyl sulfonyl fluoride (PMSF) (catalogue number, ST506-2; Beyotime, Shanghai, China) (94:6). Protein concentration was determined using a BCA protein assay kit (catalogue number 23227; Thermo Fisher Scientific, Inc.) following the manufacturer’s instructions. Proteins were separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (catalogue number P0012A; Beyotime) (10%) and then transferred to a polyvinylidene difluoride membrane (PVDF) (catalogue number FFP39; Beyotime). The membrane was immunoblotted with anti-β-actin (1:1000), anti-Sirt1 (1:500), anti-p-Sirt1 (1:250), anti-PAI-1(1:500) at 4°C overnight. Afterward, the corresponding secondary antibody (1:1000) conjugated with peroxidase and enhanced chemiluminence reagents (catalogue number, P0018; Beyotime) were applied to visualize the targeted antigens. The protein contents were assessed using the Labwork image analysis software (catalogue number P2403; Biomagin Systems Pvt. Ltd., Battaramulla, Sri Lanka).
SA-β-gal staining
SA-β-gal staining was performed to determine the effect of allicin on aging using a Senescence β-Galactosidase Staining Kit. HUVECs with allicin (5 ng/mL) and allicin (5ng/mL)+NAM or NAM for 24 h. The cells were replaced the media with 25 μM H2O2 for 1 h. The media were then replaced with fresh medium for 24 h. The cells were washed with PBS 3 times, and then HUVECs were stained for SA-β-gal activity using the Senescence β-Galactosidase Staining Kit. SA-β-gal-positive cells were photographed with a microscope, which included counting >200 cells in 3 independent fields. The percentage of SA-β-gal-positive cells was determined by counting the number of positive cells within a sample [20].
qRT-PCR
Total RNA was extracted from the tissue samples using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc. Waltham, MA, US). Subsequently, complementary DNA was synthesized using a reverse transcriptase kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturers’ instructions. The relative expression levels of mRNA were determined using a SYBR Green real-time PCR kit (Agilent Technologies, Inc., Santa Clara, CA, USA) and normalized to GAPDH. RT-PCR was performed using the ABI 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the following gene-specific primers: GAPDH sense: 5′-TGCCATCAACGACCCCTTCA-3′; GAPDH anti-sense: 5′-TGACCTTGCCCACAGCCTTG-3′; Sirt1 Sense: 5′-GAGGCAGTGCAGCATGTAGT-3′, Anti-sense 5′-GATGATTCCCTCGGTCAGAA-3′. All primers were designed using the National Center for Biotechnology Information Primer-BLAST tool. PCR was performed under the following conditions: Denaturation at 50°C for 2 min, followed by 35 cycles of 94°C for 15 s and 56°C for 45 s. Gene expression was normalized to internal controls and fold changes were calculated using relative quantification (2−ΔΔCt).
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM) or mean ± standard deviation (SD). Differences between groups were evaluated using analysis of Student’s t test or analysis of variance (ANOVA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of allicin on HUVEC viability
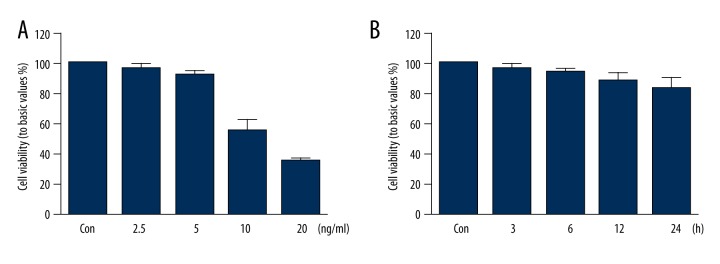
Previous studies reported that high concentrations of allicin could inhibit its effects on cell viability [14]; thus, we investigated the effect of allicin on HUVEC viability. As shown in Figure 1, 2.5 and 5 ng/mL allicin did not affect the viability of HUVECs, whereas 10 ng/mL and 20 ng/mL allicin inhibited the viability of HUVECs significantly (P<0.05) (Figure 1A). Therefore, we selected 5 ng/mL allicin for the subsequent experiment because this concentration did not influence HUVEC viability. HUVECs were then treated with 5 ng/mL allicin at different times (0, 3, 6, 12, and 24 h) to investigate further the influence of 5 ng/mL allicin on HUVEC viability. The results in Figure 1B show that 5 ng/mL allicin did not affect the viability of HUVECs at different times.
Figure 1.
Effect of allicin on HUVEC viability: (A) HUVECs incubated with different concentrations of allicin (2.5, 5, 10, and 20 ng/mL) for 12 h; (B) HUVECs treated with 5 ng/mL allicin at different times (0, 3, 6, 12, and 24 h). Cell viability detected by thiazole blue chromatometry. Data derived from 3 different experiments and presented in mean ±SD (n=3); * P<0.05 compared with the control group.
Effects of allicin on ROS production and H2O2-induced HUVEC aging
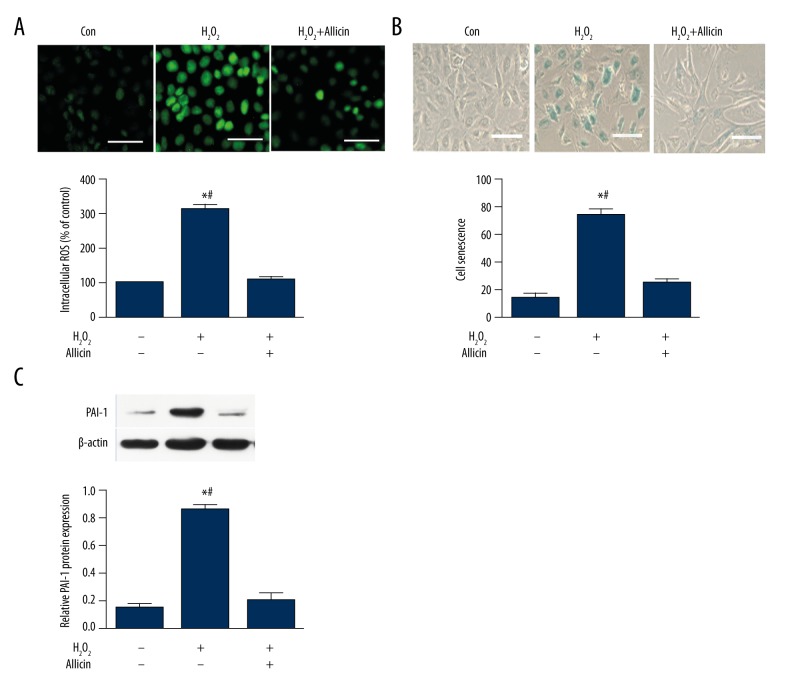
ROS is an important factor affecting endothelial cells; thus, the effect of allicin on the ROS level in HUVECs is investigated. We treated HUVECs with 25 μmol/L H2O2 before 5 ng/mL allicin treatment. H2O2 significantly enhanced the generation of cellular ROS compared with the control. However, the increase in ROS was attenuated by allicin treatment (Figure 2A).
Figure 2.
Effects of allicin on ROS production and H2O2-induced HUVEC aging. (A) Effect of allicin on H2O2-induced ROS production in HUVECs. Cells were pretreated with 25 μmol/L H2O2 for 1 h and then incubated with 5 ng/mL allicin for 24 h, levels of the fluorogenic probe DCFH-DA were determined to analyze ROS formation. Data were derived from 3 independent experiments and are in mean ±SD (n=3); * P<0.05 compared with the control group; # P<0.05 compared with the H2O2+allicin group. (B) Effect of allicin on H2O2-induced HUVEC aging. Cells were exposed to 25 μmol/L H2O2 and then incubated with 5 ng/mL allicin for 24 h. Cell aging was detected by senescence-associated β-galactosidase (SA-β-gal) staining. Data were derived from 3 independent experiments and are in mean ±SD (n=3); * P<0.05 compared with the control group; # P<0.05 compared with the H2O2+allicin group. (C) PAI-1 protein was analyzed by Western blot. Data were derived from 3 independent experiments and are in mean ±SD (n=3); * P<0.05 compared with the control group; # P<0.05 compared with the H2O2+allicin group.
A number of studies have demonstrated that ROS promotes cell aging [15]. In this study, whether allicin attenuates H2O2-induced HUVEC aging was investigated. HUVECs were treated with H2O2 in the presence or absence of allicin. Senescence-associated β-galactosidase (SA-β-gal) staining results demonstrated that allicin significantly suppressed H2O2-induced cell aging (Figure 2B). Moreover, we also detected the protein expression associated with aging by Western blot. Our results showed that allicin significantly inhibited H2O2-induced PAI-1 protein expression, suggesting that allicin reduced ROS production and inhibited H2O2-induced HUVEC aging (Figure 2C).
Effect of allicin on H2O2-induced Sirt1 activation in HUVECs
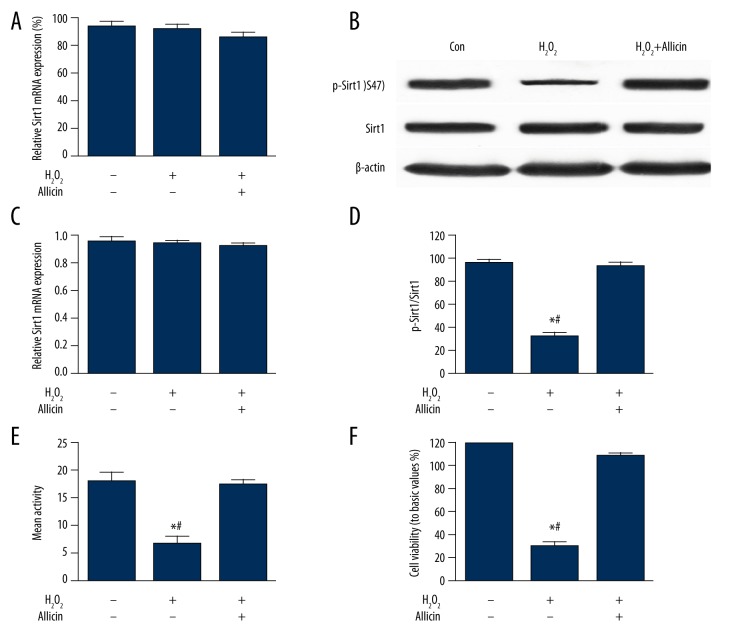
Sirt1 regulates and delays cell aging, suppresses apoptosis, and regulates metabolism (e.g., calorie intake and fat storage) [16,17]. In this study, we also investigated whether Sirt1 is involved in the inhibitory effect of allicin on H2O2-induced HUVEC aging. First, HUVECs were exposed to 25 μmol/L H2O2 and then incubated with 5 ng/mL allicin for 24 h. As shown in Figure 3, the expression of p-Sirt1 protein was reduced significantly in the H2O2 group, and Sirt1 activity and cell viability decreased (P<0.05) compared with the control group. However, these effects were reversed after allicin treatment. In addition, the expression levels of Sirt1 mRNA and protein did not change in this study, indicating that Sirt1 could be involved in the inhibitory effect of allicin on H2O2-induced HUVEC aging.
Figure 3.
Effect of allicin on H2O2-induced Sirt1 expression and activity. HUVECs were exposed to 25 μmol/L H2O2 and then incubated with 5 ng/mL allicin for 24 h. (A) Expression of Sirt1 mRNA was analyzed by real-time PCR. (B–D) Expression levels of Sirt1 and p-Sirt1 protein were determined by Western blot. (E) Sirt1 deacetylase activity was reduced in the group treated with 25 μmol/L H2O2, and the addition of 5 ng/mL allicin returned the activity to control levels. (F) HUVECs were exposed to H2O2 (25 μmol/L) and then incubated with 5 ng/mL allicin for 24 h. Cell viability was detected by thiazole blue chromatometry. Data are in mean ±SD (n=3); * P<0.05 compared with the control group; # P<0.05 compared with the H2O2+allicin group.
Reversal of anti-aging effect of allicin on HUVECs by NAM
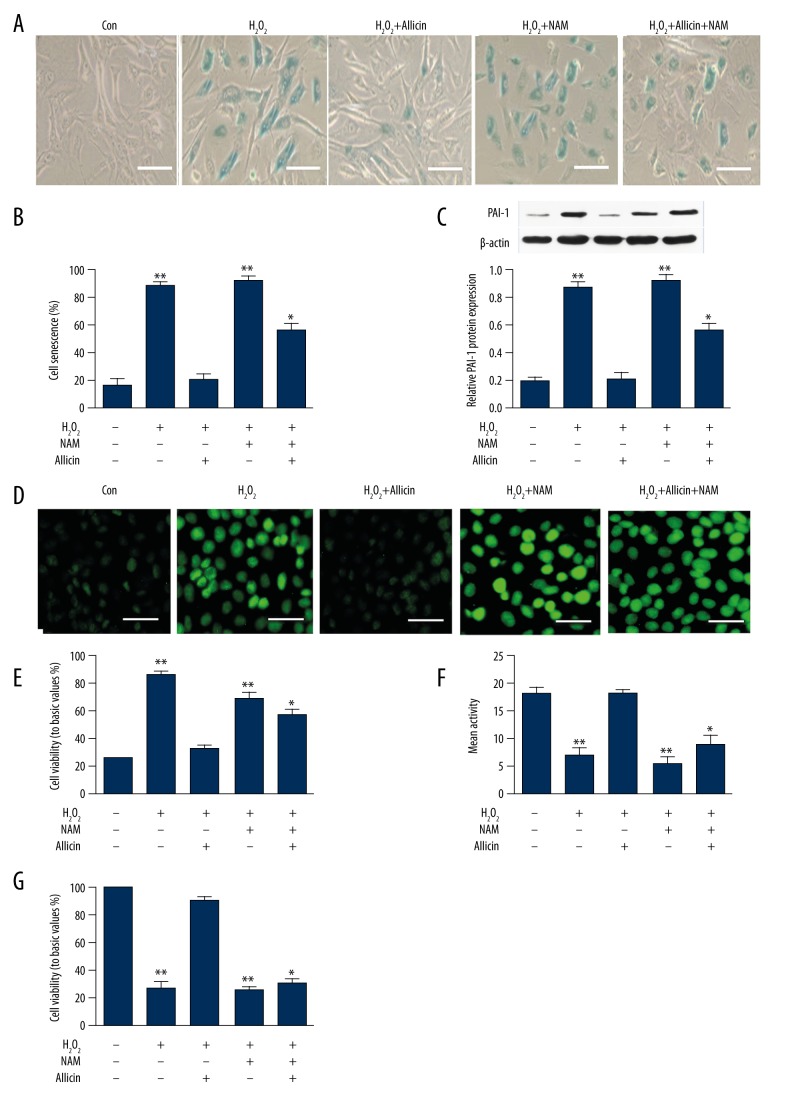
The results presented in Section 2.3 suggest that allicin can induce Sirt1 activity. Thus, to investigate whether Sirt1 activation is involved in the inhibitory effect of allicin on H2O2-induced HUVEC aging, we treated HUVECs with 25 μmol/L H2O2 to establish the aging model. Subsequently, we treated the same HUVECs with allicin (5 ng/mL) and allicin (5 ng/mL)+NAM or NAM only for 2 h. Allicin significantly inhibited cell aging and cellular ROS generation in HUVECs compared with the control group (Figure 4A, 4B). However, these effects were attenuated by the addition of NAM. Moreover, allicin increased the expression of PAI-1 protein, and Sirt1 activity was suppressed by NAM treatment (Figure 4C, 4D). In addition, cell viability was also reduced by NAM co-treatment (Figure 4E). Thus, H2O2-induced HUVEC aging depended on the activation of Sirt1.
Figure 4.
Effect of allicin on the H2O2-induced senescence of HUVECs with inhibited Sirt1 expression. HUVECs were exposed to 25 μmol/L H2O2, incubated with NAM for 2 h, and then treated with 5 ng/mL allicin for 24 h. (A, B) Cell senescence were detected by SA-β-gal staining. (C) Expression of PAI-1 protein was analyzed by Western blot. (D, E) Levels of the fluorogenic probe DCFH-DA were determined to analyze ROS formation. (F) Sirt1 deacetylase activity was analyzed with an ELISA kit. (G) Cell viability was detected by thiazole blue chromatometry. Data were derived from 3 independent experiments and are in mean ±SD (n=3); * P<0.05 and ** P<0.01 compared with H2O2+allicin group.
Discussion
Atherosclerosis is the pathological basis of various types of cardio-cerebrovascular diseases. Vascular endothelial damage and dysfunction are an initiating step of atherosclerosis, and senility of endothelial cell is one of the primary causes for impaired endothelial function. Therefore, research and development of drugs for anti-senility of endothelial cell are greatly significant in preventing and treating atherosclerosis, even in treatment of cardio-cerebrovascular diseases. Allicin, as a natural plant extract, possesses preventive and therapeutic effects on cardiovascular diseases. It has attracted increasing attention. Studies have shown that allicin has multiple effects, including anti-inflammation, anti-oxidation, and lipid regulation, as well as potential for preventing atherosclerosis. However, the specific mechanisms remain unclear [1,2]. The results of the present study show that allicin can reduce the protein expression level of PAI-1 and decrease the amount of SA-β-gal positive cells (i.e., HUVEC senility can be inhibited by allicin). In addition, Sirt1 activity can be significantly upregulated by allicin to reduce the ROS production in cells, thereby playing an anti-senility role in cells.
ROS accumulation produced by oxidative stress in cells is known as an important cause of cell damage and senility [18]. Many studies have demonstrated that vascular endothelial cells can be stimulated by the ROS generated in vessel walls to produce various types of inflammatory factors (e.g., IL-6 and TNF-α) involved in the development and progression of atherosclerosis [19,20]. Therefore, in vivo ROS production and scavenging are the important mechanisms for atherosclerosis prevention. Studies have also demonstrated that ROS affects the activation of protein kinase B (PKB), mitogen-activated protein kinase (MAPK), and nuclear transcription factor kappa B (NF-κB) signaling pathways, regulates endothelial nitric oxide synthase (eNOS) expression in endothelial cells and nitric oxide (NO) production, and influences endothelial function [21,22]. The present study shows that the ROS production in HUVECs can be significantly facilitated by hydrogen dioxide to accelerate cell senility and promote PAI-1 expression, which are consistent with the study results of Suo Rong [13]. However, the effect of H2O2 on HUVECs is reversed after cell co-treatment with allicin and H2O2. The above-mentioned results show that allicin can protect HUVECs against H2O2-induced senility by inhibiting ROS production.
Sirt1 is a key protein for cell senility regulation; thus, upregulating Sirt1 activity can significantly inhibit cell senility. Many studies have shown that Sirt1 plays an important role in anti-senility. Cell senility process can be regulated by regulating forkhead box O (FOXO), P73, and Ku60 [23–25]. Sirt1 can deacetylate for FOXO3, enhance gene transcription for superoxide dismutase (SOD) and catalase, and resist the senility of mouse embryonic fibroblast induced by oxidative stress [26]. Sirt1 is speculated to be an important senility regulation factor, and its function is affected by the expression level and activity of itself [27]. However, the mechanism for regulating the Sirt1 activity is unknown. Suo Rong [13] reported that H2S2 facilitates Sirt1 activation in endothelial cells and antagonizes the impairment of these cells induced by oxidative stress. Wu [28] showed that ROS production in endothelial cells can be reduced by upregulating the Sirt1 activity to subsequently protect vascular endothelial cells against ROS injury. This result suggests that the increase in Sirt1 activity has the potential to inhibit ROS and the ability to antagonize endothelium impairment induced by oxidative stress. In the current research, our study team found that allicin facilitates Sirt1 phosphorylation and upregulates Sirt1 activity. Accordingly, the ROS production and PAI-1 expression level in the cells can be reduced by allicin. These results are similar to those of Wu and Suo Rong. Reduction of ROS production and viability enhancement for endothelial cells can also be induced by Sirt1 activation. After treating HUVECs with NAM, which is an inhibitor for Sirt1 activation, the resistance of allicin against ROS production and the decrease in viability and senility for HUVECs are all reversed. Meanwhile, the Sirt1 phosphorylation is inhibited, and the PAI-1 protein expression level associated with senility is significantly increased. These results show that allicin increases cell viability and plays an anti-senility role by activating Sirt1 to reduce ROS production. However, the issues about the relationship between Sirt1 inhibition on ROS production and PAI-1 protein expression, as well as whether NAM plays the role of anti-senility by inhibiting Sirt1 phosphorylation, are not included in this article. These topics need further in-depth study in the future.
Conclusions
Our results revealed that allicin reverses H2O2-induced HUVEC aging, and it inhibits the production of ROS by activating Sirt1. This study provides new evidence that allicin has a role in prevention of endothelial cell aging, and Sirt1 is a regulator of intracellular ROS, suggesting that Sirt1 activation is may be used as a novel therapeutic strategy to protected against H2O2-induced HUVEC aging.
Footnotes
Source of support: This study was supported by the Medical Foundation of Hui Zhou (2015Y134); the Medical Research Foundation of Guangdong Province (A2015620); and the Graduate Student Research Innovation Project of Hunan Province (CX2013B396)
References
- 1.Song Z, Liu Y, Hao B, et al. Ginsenoside Rb1 prevents H2O2-induced HUVEC senescence by stimulating Sirtuin-1 pathway. PLoS One. 2014;9(11):1–11. doi: 10.1371/journal.pone.0112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo XY, Qu SL, Tang ZH, et al. Sirt1 in cardiovascular aging. Clin Chim Acta. 2014;437(4):106–14. doi: 10.1016/j.cca.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Bayan L, Koulivand PH, Gorji A. Garlic: A review of potential therapeutic effects. Avicenna J Phytomed. 2014;4(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- 4.Ginter E, Simko V. Garlic (Allium sativum L.) and cardiovascular diseases. Bratisl Lek Listy. 2010;111(8):452–56. [PubMed] [Google Scholar]
- 5.Li RK, Li JF, Zhou XR, et al. Effects of allicin on plasma lipid metabolism of atherosclerotic mice. Chin J Clin(Electronic Version) 2007;1(1):29–33. [Google Scholar]
- 6.Li H, Horke S, Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237(1):208–19. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Qi H, Wang Y, et al. Allicin protects against myocardial apoptosis and fibrosis in streptozotocin-induced diabetic rats. Phytomedicine. 2012;19(8–9):693–98. doi: 10.1016/j.phymed.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Chan JY, Yuen AC, Chan RY, et al. A review of the cardiovascular benefits and antioxidant properties of allicin. Phytother Res. 2013;27(5):637–46. doi: 10.1002/ptr.4796. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira RM, Pais TF, Outeiro TF. Sirtuins: Common targets in aging and in neurodegeneration. Curr Drug Targets. 2010;11(10):1270–80. doi: 10.2174/1389450111007011270. [DOI] [PubMed] [Google Scholar]
- 10.Poulose N, Raju R. Sirtuin regulation in aging and injury. Biochim Biophys Acta. 2015;1852(11):2442–55. doi: 10.1016/j.bbadis.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong H, Cohen DE, Cui L, et al. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2011;18(1):159–65. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang QJ, Wang Z, Chen HZ, et al. Endothelium-specific overexpression of class III deacetylase Sirt1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80(2):191–99. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suo R, Zhao ZZ, Tang ZH, et al. Hydrogen sulfide prevents H2O2-induced senescence in human umbilical vein endothelial cells through Sirt1 activation. Mol Med Rep. 2013;7(6):1865–70. doi: 10.3892/mmr.2013.1417. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Zhang J, Zhang L, et al. Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of osteosarcoma cells by switching on suppressor microRNAs and inactivating of Notch-1 signaling. Carcinogenesis. 2013;34(7):1601–10. doi: 10.1093/carcin/bgt065. [DOI] [PubMed] [Google Scholar]
- 15.Yang SR, Park JR, Kang KS. Reactive oxygen species in mesenchymal stem cell aging: Implication to lung diseases. Oxid Med Cell Longev. 2015;2015:486263. doi: 10.1155/2015/486263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cetrullo S, D’Adamo S, Tantini B, et al. mTOR, AMPK, and Sirt1: Key players in metabolic stress management. Crit Rev Eukaryot Gene Expr. 2015;25(1):59–75. doi: 10.1615/critreveukaryotgeneexpr.2015012975. [DOI] [PubMed] [Google Scholar]
- 17.Chang HC, Guarente L. Sirt1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25(3):138–45. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Z, Liu S, Wang X, et al. LOX-1, oxidant stress, mtDNA damage, autophagy, and immune response in atherosclerosis. Can J Physiol Pharmacol. 2014;92(7):524–30. doi: 10.1139/cjpp-2013-0420. [DOI] [PubMed] [Google Scholar]
- 19.Vara D, Pula G. Reactive oxygen species: physiological roles in the regulation of vascular cells. Curr Mol Med. 2014;14(9):1103–25. doi: 10.2174/1566524014666140603114010. [DOI] [PubMed] [Google Scholar]
- 20.Lubrano V, Balzan S. LOX-1 and ROS, inseparable factors in the process of endothelial damage. Free Radic Res. 2014;48(8):841–48. doi: 10.3109/10715762.2014.929122. [DOI] [PubMed] [Google Scholar]
- 21.Li XL, Liu JX, Li P, et al. [Protective effect of rosmarinic acid on hypoxia/reoxygenation injury in cardiomyocytes]. Zhongguo Zhong Yao Za Zhi. 2014;39(10):1897–901. [in Chinese] [PubMed] [Google Scholar]
- 22.Yuan L, Lu CL, Wang Y, et al. Ang (1–7) protects islet endothelial cells from palmitate-induced apoptosis by AKT, eNOS, p38 MAPK, and JNK pathways. J Diabetes Res. 2014;14(5):1–10. doi: 10.1155/2014/391476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchiya K, Tanaka J, Shuiqing Y, et al. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15(3):372–81. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponugoti B, Dong G, Graves DT. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res. 2012;7(4):939751–58. doi: 10.1155/2012/939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi LS, Yao L, Liu W, et al. Sirtuin type 1 mediates the retinal protective effect of hydrogen-rich saline against light-induced damage in rats. Invest Ophthalmol Vis Sci. 2015;56(13):8268–79. doi: 10.1167/iovs.15-17034. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107(2):137–48. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 27.Milner J. Cellular regulation of Sirt1. Curr Pharm Des. 2009;15(1):39–44. doi: 10.2174/138161209787185841. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Xia S, Kalionis B, et al. The role of oxidative stress and inflammation in cardiovascular aging. Biomed Res Int. 2014;2014(7):1–11. doi: 10.1155/2014/615312. [DOI] [PMC free article] [PubMed] [Google Scholar]