Abstract
Purpose
In vitro data demonstrate that heat-induced radiosensitisation is maximised if hyperthermia and radiotherapy are given simultaneously, with the radiation fraction delivered midway through a hyperthermia session, rather than sequentially. The long-term normal tissue toxicity of full-dose simultaneous thermoradiotherapy is unknown.
Materials and methods
Patients with locally advanced breast cancer (T3, T4 or more than three involved nodes or local recurrence), no prior radiotherapy, received between four and eight sessions of simultaneous thermoradiotherapy. Hyperthermia always included the primary tumour site. In addition an electively heated sector (EHS) was included. The EHS was randomised to either medial or lateral to the tumour site, with the other side an irradiated but unheated control. As per our usual practice, patients received surgery and/or chemotherapy prior to radiotherapy. Radiation doses were 46–50 Gy followed by a boost of ≤16 Gy at 1.8–2 Gy per fraction. EHS and control sectors received the same dose.
Results
A total of 57 evaluable cases with average follow-up of 79 months experienced two local and two nodal recurrences. There was no significant difference in ≥grade 2 toxicity for heated versus control sectors (LR χ2 = 0.78, p = 0.38) with no relationship between number of hyperthermia sessions and toxicity (LR χ2 = 2.90, p = 0.09).
Conclusions
Simultaneous full-dose thermoradiotherapy for breast cancer is feasible and well tolerated, with no significant difference in late toxicity between electively heated and unheated control sectors. All patients had hyperthermia to the primary tumour site with excellent local control.
Keywords: Breast carcinoma, elective hyperthermia, simultaneous thermoradiotherapy, superficial hyperthermia, ultrasound hyperthermia
Introduction
Hyperthermia is a well-validated adjunct to radiotherapy for the treatment of breast cancer [1, 2]. Breast cancer in the chest wall, in particular, tends to be broad but very shallow, making it very suitable for heating with modern microwave or ultrasound array devices. However, the principal use to date has been for treatment of patients with macroscopic disease, most commonly local recurrences after prior radiotherapy and systemic therapy. Although such cases are important, they represent a minority of breast cancer patients referred for radiotherapy that may also benefit from hyperthermia. Because the range of systemic treatments is increasing, patients with local recurrence tend to go through a number of systemic therapies before being considered for re-irradiation, and therefore hyperthermia referrals are much less frequent than in the past. Moreover, patients with locally advanced initial presentation of disease – who do comprise a substantial group in a typical radiation oncology practice – usually receive surgery and/or chemotherapy before radiation therapy and often have no visible disease by the time they begin radiotherapy. Thus a typical radiation oncology practice, while including substantial numbers of patients with breast cancer, will have relatively few with macroscopic disease. There is reason to investigate the safety and efficacy of hyperthermia as an enhancer of radiotherapy for patients with high risk of local failure in areas with no visible disease at the time of treatment. Several publications have suggested that hyperthermia with low-dose re-irradiation can achieve good local control for patients who have had all macroscopic disease eradicated prior to radiotherapy. [3–5]. In the present study we evaluate elective thermoradiotherapy for patients who have had no prior radiotherapy.
In management of subclinical disease, one of the major rationales for applying hyperthermia – its effect on tumour physiology – probably does not apply. A number of investigators have shown that readily achievable minimum target thermal doses of ~41°C × 30–60 min can produce substantial reductions of tumour hypoxia [6–10]. However, for electively treated regions that have seen no prior radiotherapy, tumour micrometastases share the physiology of the normal tissue. Normoxic tumour cells can still benefit from hyperthermia via the mechanism of heat-induced radiosensitisation (HIR). In this scenario, the rationale for simultaneous hyperthermia and radiation is buttressed. HIR has been shown to depend sharply on timing: if radiation is given during hyperthermia (simultaneous thermoradiotherapy), 1 h at 41°C produces a level of HIR comparable to 2 h or more if radiotherapy immediately follows hyperthermia (sequential thermoradiotherapy) [11–14]. We have previously shown that biologically meaningful thermal doses to tumour tissue could be achieved with four or more simultaneous hyperthermia sessions incorporated into a course of fractionated radiotherapy [12, 15].
If the target volume is being electively treated there is potentially dose-limiting normal tissue admixed with tumour micrometastases. The question of therapeutic gain is very important in this setting. To help evaluate this we have conducted a prospective clinical trial in which we compare post-treatment normal tissue changes in heated versus unheated portions of electively treated chest walls or breasts. Each woman functioned as her own control; with the heated sector randomised to be either medial or lateral to the location of the primary tumour, as described in the next section. This protocol also included a thermal dose escalation component. The first cohort of enrolled patients received four hyperthermia sessions, followed by a second cohort that received eight. Since late injury is usually the dose-limiting factor for radiotherapy, late effects were the primary end point of this protocol.
Methods and materials
The present study was a randomised dose escalation trial of simultaneous thermoradiotherapy to the chest wall for high-risk breast cancer patients with minimal to no residual disease and no prior radiation therapy to the chest wall. Each patient served as her own control, with random selection of either the medial or lateral half of the chest wall to receive elective hyperthermia. The primary end point was late skin changes in the heated and unheated control regions during follow-up. The study protocol was approved by the Institutional Review Board of Washington University in Saint Louis.
Patient selection
Eligible patients were those with histopathologically confirmed adenocarcinoma of the breast with T4, T3, any T-stage with more than three involved lymph nodes, or locoregional recurrent disease, with no evidence of distant disease other than the ipsilateral supraclavicular region, no prior radiotherapy, and macroscopic disease on the chest wall ≤3 cm in greatest dimension at the time of initiation of radiation treatment. Patients with prior surgical resection of all visible disease or complete clinical response to prior chemotherapy were included. The eligibility criteria were based on our past experience, which indicated that even with adjuvant radiotherapy these patients have an overall risk of locoregional failure in excess of 10% [16–18]. Studies to determine absence of distant metastatic disease were chosen by the treating physician as per customary practice, and included laboratory studies (CBC (complete blood count), serum electrolytes, liver function tests) and at least one radiographic study (chest X-ray, computerized tomography (CT) scan of chest and abdomen, bone scan, or fluorodeoxyglucose positron emission tomography (FDG-PET) scan).
Patients receiving breast conservation therapy were allowed if breast depth was ≤5 cm; breasts deeper than that were excluded due to inability to adequately heat with ultrasound hyperthermia. Patients who were <18 years old or otherwise unable to give informed consent, pregnant patients, and patients with pacemakers or other implantable devices susceptible to radiofrequency interference from the device were also excluded. Eligible patients were required to have an estimated survival time ≥1 year at the time of enrolment.
Radiotherapy delivery
All patients were prescribed 46–50 Gy in 1.8–2 Gy fractions to the chest wall and lymphatics of the supraclavicular fossa and axilla. Tangents were used to treat the breast or chest wall. On hyperthermia days, the hyperthermia applicator was placed orthogonal to the radiation beam, with radiation treated midway through the hyperthermia session as described previously [19]. Boost treatment to a total dose of up to 66 Gy was permitted, provided that the dose to both the heated and control sectors was the same. In general 6 MV photons were used to treat tangents, except for patients with bridge separation ≥25 cm, who were permitted to receive up to 14 fractions with higher energy. Layer bolus to the chest wall was required at least every other day. Since the hyperthermia applicator functions as bolus to the heated sector, hyperthermia was always scheduled on days when the unheated control sector was also being bolused.
In addition to the usual skin tattoos for treatment set-up, an additional triangle of permanent tattoos was placed in the heated and control sectors to help define the extent of tissue contraction in follow-up. The ratio of follow-up to initial values of the product of the base times the height of each triangle was used as a measure of tissue contracture: the smaller the ratio, the greater the amount of contracture.
Hyperthermia delivery
Hyperthermia was delivered simultaneously with radiation, in 1-h sessions. Hyperthermia treatment took place in the radiation treatment room with the patient positioned for radiation treatment. The radiation fraction was delivered midway through the 60 min hyperthermia treatment, with no interruption of the hyperthermia. Techniques for simultaneous thermoradiotherapy were as per Straube et al. [19].
Hyperthermia was delivered via the Sonotherm 1000 ultrasound array system, using 16 independent transducers (Labthermics, Champaign, IL, USA). Treatments were delivered using the 16 cm × 16 cm aperture, which effectively heats a 12 cm × 12 cm area [20]. The primary tumour site was always included in half the hyperthermia field, with patients randomised to have the other half of the hyperthermia field (elective hyperthermia) lie either medial (27 cases) or lateral (30 cases) to the primary site. The electively heated hyperthermia sector was thus 6 × 12 cm. An unheated control sector, also 6 × 12 cm, lay on the other side of the primary lesion and matched the heated sector in cephalocaudal extent. To make certain that the control sector was not heated by conduction, a gap of 5 cm was required between the hyperthermia applicator coupling bolus and the control sector.
A minimum of 72 h between hyperthermia sessions was required. Two study arms were defined with regard to number of hyperthermia treatments: Patients on Arm 1 received four hyperthermia treatments, in most cases once a week during the first 4 weeks of therapy. Patients on Arm 2 received eight hyperthermia treatments, preferably within the first 4 weeks of radiotherapy. Arm 2 was not opened until at least 10 patients with a minimum of 1.5 years follow-up were accrued.
Hyperthermia sessions lasted about 60 min, with a thermal objective of heating all monitored locations to at least 41°C for at least 30 min, while limiting maximum skin temperature to 43°C as much as possible. Continuous temperature monitoring was done using thermocouples in multi-sensor surface and interstitial probes. The number of monitored locations used per hyperthermia session ranged from 11 to 16, with a mean of 15.1 continuously monitored locations. Only one patient had macroscopic residual disease at the initiation of radiotherapy, a small area of skin nodularity <1 cm thick, monitored with surface sensors. For all other patients there was no visible disease after the pre-radiation chemotherapy and/or surgery. Interstitial thermometry was not used if the volume at risk was less than 1 cm deep which, in this study, meant those patients with prior mastectomy. It should be noted that the coupling water bolus, although warmed to less than 41°C prior to treatment, was not actively heated during the hyperthermia sessions, and was passive (non-circulating). The ultrasound device itself deposits no power in the degassed water. Therefore elevated temperatures above 41°C in surface sensors do not simply reflect the temperature of the coupling bolus temperature. Typical tissue perfusion and conduction imply a thermal diffusion length of ~1 cm [21]. The seven patients with intact breasts had one or two three-sensor interstitial thermocouple needles placed each session, under ultrasound guidance to assure reproducibility of position. In-dwelling catheters were not used, to avoid temperature artefact during ultrasound heating.
Post-treatment follow-up
Patients were scheduled to be seen in follow-up 1 month after the completion of therapy and every 6 months thereafter. At each follow-up visit the hyperthermia physicist identified the sectors to the physician using pre-treatment diagrams and photos, but kept the scoring physician blinded as to which sector was heated and which was not. Skin changes in the heated and non-heated control sectors were evaluated by a visual scale based on the RTOG LENT system [22]. Those patients who retained skin tattoos in follow-up also had tissue contraction measured by comparing the area enclosed by the triangular array of tattoos in each sector to its pre-treatment value.
Locoregional disease status was assessed clinically. Since the primary end points of this study were normal tissue and tumour status in the chest wall, laboratory and radiographic studies were not protocol constrained but were obtained as per customary clinical practice.
Statistics and data analysis
The accrual objective was to acquire enough cases to discriminate a 15% difference in late morbidity with four hyperthermia sessions and 30% with eight hyperthermia sessions. The intention was to accrue 30 cases receiving four hyperthermia treatments and 30 receiving eight. However, rate of accrual to Arm 1, although averaging better than one a month, was initially slow. Arm 2 was not allowed to begin until both the total number of evaluable cases on Arm 1 reached 30 and there were at least 10 patients with at least 1.5 years follow-up. Because of the accelerating accrual rate and the need for adequate follow-up of the Arm 1 patients to confirm no unexpectedly high complication rates prior to opening Arm 2, we actually concluded the study with 52 patients on Arm 1 (four hyperthermia sessions) and seven patients on Arm 2 (eight hyperthermia sessions), accrued.
Kaplan-Meier plots and Cox proportional hazards regression modelling were used to investigate associations of overall survival time, survival time with no evidence of disease, time to locoregional failure, and time to ≥grade 2 toxicity with clinical variables. Clinical variables of interest included patient age, RT dose, number of hyperthermia treatments, thermal dose, and clinical scenario (initial versus recurrent disease presentation).
Statistical analyses were performed using SYSTAT 8.0 and SAS v. 9. for Windows (SPSS, Chicago, IL). The cut-off for statistical significance was set at an unadjusted alpha level of 0.05.
Results
A total of 59 patients enrolled in the protocol, 52 on Arm 1, receiving four hyperthermia treatments, and seven on Arm 2, receiving eight hyperthermia treatments. Two patients (on Arm 1) subsequently withdrew consent and received no hyperthermia (both of whom succumbed to cancer), leaving 57 evaluable cases. Demographic and clinical characteristics of the patients are summarised in Table I. For patients NED (no evidence of disease) at last follow-up, the mean follow-up time was 79 months (median 86 months), with a range from 19 to 120 months.
Table I.
Characteristics of 57 evaluable subjects.
| Number or mean ± SD | |
|---|---|
| Age (years) | 52.5 ± 11.1 |
| Pre-radiotherapy treatment | |
| Surgery | 56 |
| Chemotherapy | 55 |
| Clinical scenario | |
| Initial presentation | 49 |
| T1–2, N > 1 | 25 |
| T3–4, any N | 24 |
| Local recurrence, no prior RT | 8 |
| Disease at start of RT | |
| No visible disease | 56 |
| Macroscopic disease | 1 |
| Receptor status | |
| ER−, PR−, HER2neu− | 12 |
| ER−, PR−, HER2neu+ | 6 |
| ER or PR+, HER2neu− | 22 |
| ER or PR+, HER2neu+ | 17 |
| Radiation dose (Gy) to heated and control sectors | |
| <60 Gy (51.8 ± 6.1 Gy) | 23 |
| ≥60 Gy (62.1 ± 2.4 Gy) | 34 |
SD, standard deviation; RT, radiotherapy; ER, oestrogen receptor; PR, progesterone receptor; HER2neu, human epidermal growth factor receptor 2
Survival and locoregional control
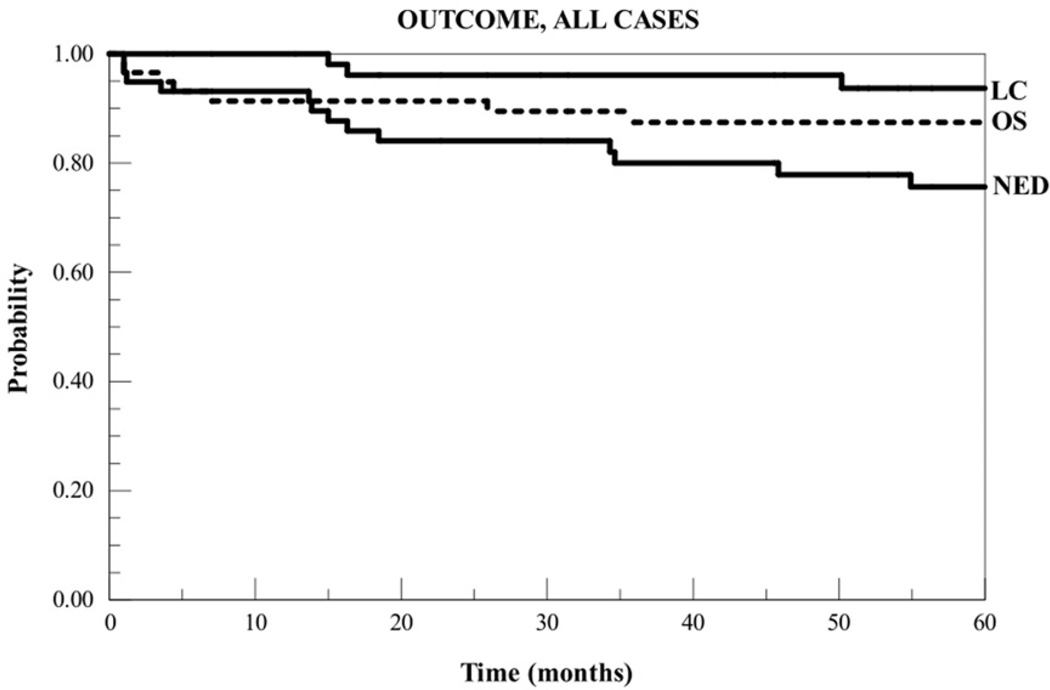
During the follow-up period, there were eight deaths, two of which resulted from intercurrent causes. Of 57 evaluable cases, 17 (30%) experienced disease recurrence, including four (7%) with locoregional recurrence and 16 with distant failure (one case failed regionally but has yet to develop distant metastases in four years of subsequent follow-up). Of the four locoregional recurrences, two (3.5%) were local in the chest wall and two were in regional nodes with the chest wall locally controlled. The two chest wall recurrences were both diffuse, involving both the heated and control sectors. Kaplan-Meier curves for overall survival, survival with no evidence of disease, and locoregional control are shown in Figure 1.
Figure 1.
Kaplan-Meier curves for overall survival (OS), freedom from disease relapse (NED), and locoregional control (LC) for all subjects.
Univariate and multivariate analyses showed that neither locoregional failure nor overall failure was significantly associated with age, RT dose, number of hyperthermia treatments, receptor status, or clinical scenario (initial presentation versus recurrence). Of potential co-factors for outcome, receptor status was the closest to significant: tumours that were ER− PR− did worse with locoregional recurrences occurring in 3/18 = 16.7% of cases (including 2/12 HER2neu− cases and 1/6 HER2neu + cases) versus 1/39 = 2.6% of cases that were either ER+ or PR+ and disease recurrence at any site occurring in 6/18 = 33% ER− PR− cases (including 4/12 triple negative cases) versus 11/39 = 28% in the remainder. Neither achieved statistical significance.
Acute and late morbidity
At completion of thermoradiotherapy 25 patients had moist desquamation in the chest wall. Of these patients, 21 had moist desquamation in both the heated and control sectors, three in only the heated sector, and one in only the control sector. This healed for all cases and is not counted as late morbidity.
Beyond 3 months post radiation, 35 patients developed late ≥grade 2 morbidity in the chest wall, with eight of these cases being ≥grade 3. The majority of ≥grade 2 late morbidities (23 cases) were asymptomatic pigment changes. Other late morbidities included sensation abnormalities (17 cases), fibrosis (12 cases), telangiectasias (10 cases), and scaliness/roughness (two cases). No cases of ≥grade 2 ulceration were observed.
Influence of hyperthermia and radiation dose on late morbidity
To evaluate thermal dose we calculated the Sapareto-Dewey [23] equivalent minutes at 43°C recorded by each temperature sensor at each hyperthermia session. For each patient the cumulative equivalent minutes CEM43°C90, CEM43°C50, and CEM 43°C10 were then defined as the sum of all sessions of the single session equivalent minutes which lay above 90%, 50%, and 10%, respectively, of that session’s monitored locations. The average ± standard deviation for CEM43°C90, CEM43°C50, and CEM43°C10 were 15 ± 10 min, 56 ± 30 minutes, and 169 ± 130 min respectively. Heating of interstitially placed sensors was comparable to the overall group, with an average CEM43°C90 = 23 ± 11 min. The CEM43°C may be converted to CEM41°C by multiplying by 16. Thus, the per-session CEM41°C90 was well above 30 min.
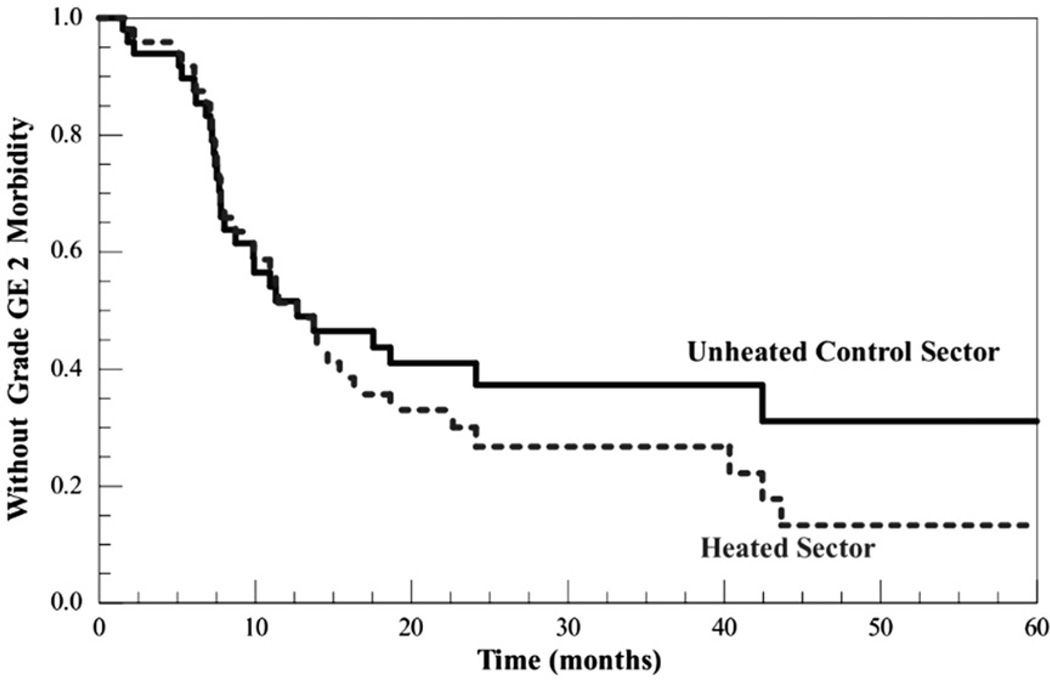
Of 35 cases with ≥grade 2 late chest wall morbidity, there were nine in one chest wall sector but not the other (all but one were grade 2): in six the sole involved sector was the heated sector, in three it was the control. Kaplan-Meier curves for freedom from ≥grade 2 morbidity in the heated and unheated control sectors, respectively, are shown in Figure 2. There was no statistically significant difference between the heated and control sectors in time to development of ≥grade 2 morbidity (log-rank χ2 = 0.78, df = 1, p = 0.38). There was no relationship between time free of ≥grade 2 morbidity in the heated sector of the chest wall and either total thermal dose (whether expressed as the CEM43°C90, CEM43°C50, or CEM43°C10) or total number of hyperthermia sessions (eight versus four). Of the seven patients who received eight hyperthermia sessions, only two experienced ≥grade 2 chest wall morbidity (one grade 2 in the heated area but not control, and one grade 3 in both the heated and control areas). The mean follow-up for these cases, since they accrued last, is shorter than the overall group but still substantial: 40 ± 3 months.
Figure 2.
Kaplan-Meier curves for freedom from grade ≥2 toxicity for heated and unheated control sectors. Separation between the curves was not statistically significant.
Mean amount of tissue contracture, expressed as the fractional change of the product of distances between orthogonal tattoos, was not statistically significantly different between heated and control sectors with means ± standard error of 0.82 ± 0.15 versus 0.91 ± 0.14, respectively. Our data for the tissue contracture measure are more limited than for the other measures. Due to patients’ refusal to allow initial tattoo placement and loss of tattoos in follow-up, these data were limited to 18 cases.
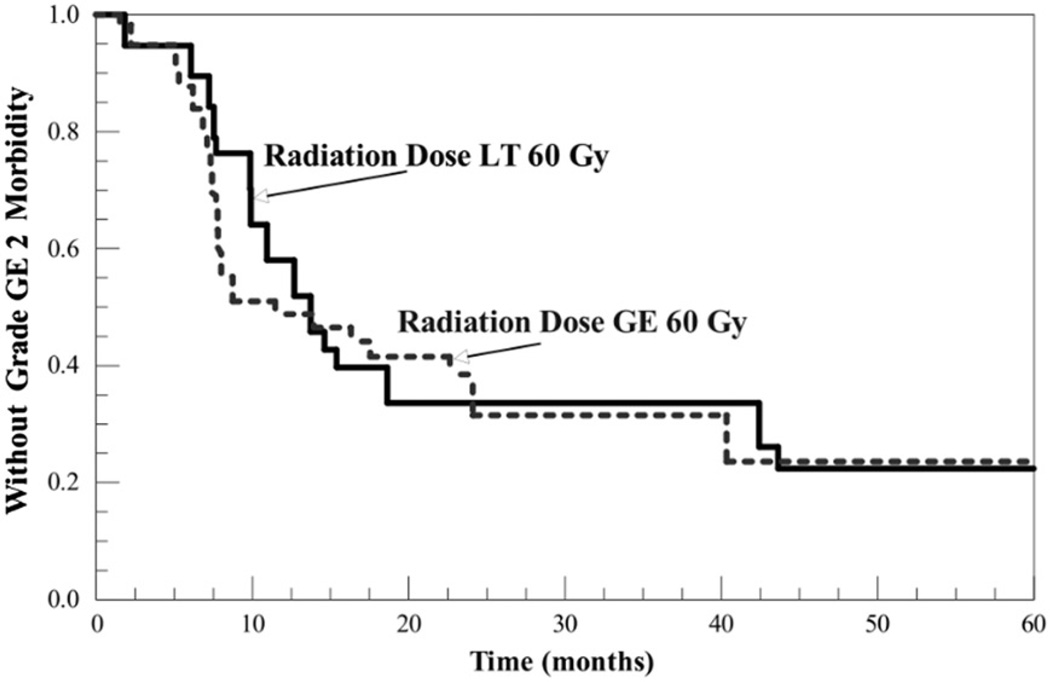
Kaplan-Meier curves for freedom from ≥grade 2 morbidity in patients receiving radiation doses less than 60 Gy (mean 51.8 ± 6.1 Gy) and those receiving greater than or equal to 60 Gy (mean 62.1 ± 2.4 Gy) are shown in Figure 3. There was no statistically significant difference between the groups (log-rank χ2 = 1.27, df = 1, p = 0.26).
Figure 3.
Kaplan-Meier curves for freedom from ≥grade 2 toxicity for patients receiving ≥60 Gy (62.1 ± 2.4 Gy) and patients receiving <60 Gy (51.8 ± 6.1 Gy). Radiation dose was at the discretion of the treating physician, but the heated and control sectors always received the same dose. Separation between the curves was not statistically significant.
Discussion
In this study elective chest wall (or breast) hyperthermia was delivered simultaneously with conventionally fractionated radiotherapy, for patients with locally advanced breast cancer and no prior radiotherapy. All patients received hyperthermia to the site of primary cancer in the chest wall and were randomised to also receive elective hyperthermia to a strip either medial or lateral to the involved site, with the opposite side functioning as an irradiated but unheated control. We therefore suggest considering our study to be a phase II efficacy evaluation as well as a phase III evaluation of late thermal normal tissue effects in the chest wall beyond the primary site. All patients received standard of care surgery and/or chemotherapy prior to their radiotherapy. All but one had no residual disease by the time they commenced radiotherapy.
The addition of hyperthermia to radiotherapy resulted in excellent locoregional control (locoregional failures: 4/57 = 7% including 2/57 = 3.5% regional nodal and 2/57 = 3.5% local chest wall). The treatments were generally well tolerated with the main late chest-wall morbidity consisting of skin discoloration/telangiectasias (no ulceration). Although there is a suggestion that the late chest-wall morbidity may be somewhat greater with hyperthermia (Figure 2), that difference did not achieve statistical significance. Moreover, except for a single case of grade 3 morbidity in the heated sector alone, the differences between heated and control sector were due to grade 2 morbidity. Thus, although it is conceivable that a much larger study than this 57-patient study might have demonstrated a statistically significant increase in grade 2 morbidity, it is highly unlikely that a grade 3 morbidity difference would be seen.
The classic study of Turesson and Notter of chest wall radiotherapy (no hyperthermia), showed that it takes about 3–5 years to establish differences between the late effects of various radiation dose fractionation schemes [24]. Thus the difference in ≥grade 2 late telangiectasias between radiation dose schemes in Turesson and Notter’s study was established by about 3 years. Although the absolute incidence of ≥grade 2 morbidity in each arm continued to increase for several years more, the differences between radiation dose schemes showed little change after 3 years (increasing but parallel curves) [24]. A subsequent paper by Turesson showed that when restricted to ≥grade 3 morbidity, the time to establish differences was somewhat longer; the different radiation dose schemes produced curves that separated and became parallel after about 5 years instead of 3 [25]. Our own data plotted in Figure 2 show a similar time dependence to Turesson and Notter [24]. In comparing the two it should be noted that the present paper reports all ≥grade 2 morbidities, whereas Turesson and Notter reported only telangiectasias. Since of the 35 cases with ≥grade 2 morbidities 10 had telangiectasias, it should not be surprising that although time dependences are similar, the absolute incidence of any morbidity reported here is ~2–3 times greater than the incidence of telangiectasias alone (curves for 2Gy × 25–30) in Figure 1 of reference 24. The mean follow-up in our study of 79 months should be sufficient, therefore, to discriminate major differences in ≤late grade 2 morbidity. Given the length of follow-up and the fact there was only a single case of grade 3 morbidity confined to the heated sector, we think it unlikely that longer follow-up would generate a large difference in ≥grade 3 morbidity – although it would be expected to show more grade 3 morbidities involving both sectors.
After establishing tolerance with four hyperthermia sessions, we treated seven patients with eight hyperthermia sessions. With a mean follow-up of 40 months, this small pilot cohort tolerated the increased number of sessions well, with one patient developing a grade 2 morbidity in the heated but not the control sector, and one patient a grade 3 morbidity in both sectors. We consider this experience sufficient to justify further exploration of eight fractions of hyperthermia for those patients with greatest risk for locoregional failure. In view of the suggestion of a greater recurrence risk for the patients with ER− PR− tumours, we would particularly recommend evaluating eight, rather than four, hyperthermia sessions for such cases.
Conclusion
Elective simultaneous thermoradiotherapy for high-risk breast cancer is feasible and well tolerated with excellent local control. Four and possibly eight hyperthermia sessions can be safely administered in the course of full dose, conventionally fractionated radiotherapy.
Acknowledgments
Declaration of interest: This study was supported by US National Institutes of Health grant R01CA71638. The authors alone are responsible for the content and writing of the paper.
References
- 1.Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys. 1996;35:731–744. doi: 10.1016/0360-3016(96)00154-x. [DOI] [PubMed] [Google Scholar]
- 2.Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23:3079–3085. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- 3.Emami B, Perez CA. Combination of surgery, irradiation, and hyperthermia in treatment of recurrences of malignant tumors. Int J Radiat Oncol Biol Phys. 1987;13:611–613. doi: 10.1016/0360-3016(87)90079-4. [DOI] [PubMed] [Google Scholar]
- 4.Kapp DS, Cox RS, Barnett TA, Ben-Yosef R. Thermoradiotherapy for residual microscopic cancer: Elective or post-excisional hyperthermia and radiation therapy in the management of local-regional recurrent breast cancer. Int J Radiat Oncol Biol Phys. 1992;24:261–277. doi: 10.1016/0360-3016(92)90681-7. [DOI] [PubMed] [Google Scholar]
- 5.Oldenborg S, Van Os RM, Van Rij CM, Crezee J, Van de Kamer JB, Rutgers EJ, et al. Elective re-irradiation and hyperthermia following resection of persistent locoregional recurrent breast cancer: A retrospective study. Int J Hyperthermia. 2010;26:136–144. doi: 10.3109/02656730903341340. [DOI] [PubMed] [Google Scholar]
- 6.Horsman MR, Overgaard J. Can mild hyperthermia improve tumour oxygenation? Int J Hyperthermia. 1997;13:141–147. doi: 10.3109/02656739709012378. [DOI] [PubMed] [Google Scholar]
- 7.Shakil A, Osborn JL, Song CW. Changes in oxygenation status and blood flow in a rat tumor model by mild temperature hyperthermia. Int J Radiat Oncol Biol Phys. 1999;43:859–865. doi: 10.1016/s0360-3016(98)00516-1. [DOI] [PubMed] [Google Scholar]
- 8.Song CW, Shakil A, Osborn JL, Iwata K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia. 1996;12:367–373. doi: 10.3109/02656739609022525. [DOI] [PubMed] [Google Scholar]
- 9.Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys. 2000;46:179–185. doi: 10.1016/s0360-3016(99)00362-4. [DOI] [PubMed] [Google Scholar]
- 10.Myerson RJ, Singh AK, Bigott HM, Cha B, Engelbach JA, Kim J, et al. Monitoring the effect of mild hyperthermia on tumour hypoxia by Cu-ATSM PET scanning. Int J Hyperthermia. 2006;22:93–115. doi: 10.1080/02656730600594191. [DOI] [PubMed] [Google Scholar]
- 11.Xu M, Myerson RJ, Straube WL, Moros EG, Lagroye I, Wang LL, et al. Radiosensitisation of heat resistant human tumour cells by 1 h at 41.1°C and its effect on DNA repair. Int J Hyperthermia. 2002;18:385–403. doi: 10.1080/02656730210146908. [DOI] [PubMed] [Google Scholar]
- 12.Myerson RJ, Roti Roti JL, Moros EG, Straube WL, Xu M. Modelling heat-induced radiosensitization: Clinical implications. Int J Hyperthermia. 2004;20:201–212. doi: 10.1080/02656730310001609353. [DOI] [PubMed] [Google Scholar]
- 13.Xu M, Myerson RJ, Hunt C, Kumar S, Moros EG, Straube WL, et al. Transfection of human tumour cells with MRE11 siRNA and the increase in radiation sensitivity and the reduction in heat-induced radiosensitisation. Int J Hyperthermia. 2004;20:157–162. doi: 10.1080/02656730310001625986. [DOI] [PubMed] [Google Scholar]
- 14.Xu M, Myerson RJ, Xia Y, Whitehead T, Moros EG, Straube WL, et al. The effects of 41°C hyperthermia on the DNA repair protein, MRE11, correlate with radiosensitisation in four human tumor cell lines. Int J Hyperthermia. 2007;23:343–351. doi: 10.1080/02656730701383007. [DOI] [PubMed] [Google Scholar]
- 15.Myerson RJ, Straube WL, Moros EG, Emami BN, Lee HK, Perez CA, et al. Simultaneous superficial hyperthermia and external radiotherapy: Report of thermal dosimetry and tolerance to treatment. Int J Hyperthermia. 1999;15:251–266. doi: 10.1080/026567399285639. [DOI] [PubMed] [Google Scholar]
- 16.Perez CA, Graham ML, Taylor ME, Levy JE, Mortimer JE, Philpott GW, et al. Management of locally advanced carcinoma of the breast, I. Noninflammatory. Cancer. 1994;74:453–465. doi: 10.1002/cncr.2820741335. [DOI] [PubMed] [Google Scholar]
- 17.Perez CA, Fields JN, Fracasso PM, Philpott GW, Soares RL, Taylor ME, et al. Management of locally advanced carcinoma of the breast, II. Inflammatory carcinoma. Cancer. 1994;74:466–476. doi: 10.1002/cncr.2820741336. [DOI] [PubMed] [Google Scholar]
- 18.Taylor ME, Perez CA, Levitt SH. Breast: Locally advanced (T3 and T4), inflammatory, and recurrent tumors. In: Perez CA, Brady LW, editors. Principles and Practice of Radiation Oncology. 3rd. New York, NY: Lippencott-Raven; 1997. pp. 1415–1448. [Google Scholar]
- 19.Straube WL, Moros EG, Low DA, Klein EE, Willcut VM, Myerson RJ. An ultrasound system for simultaneous ultrasound hyperthermia and photon beam irradiation. Int J Radiat Oncol Biol Phys. 1996;36:1189–1200. doi: 10.1016/s0360-3016(96)00369-0. [DOI] [PubMed] [Google Scholar]
- 20.Moros EG, Myerson RJ, Straube WL. Aperture size to therapeutic volume relation for a multielement ultrasound system: Determination of applicator adequacy for superficial hyperthermia. Med Phys. 1993;20:1399–1409. doi: 10.1118/1.597125. [DOI] [PubMed] [Google Scholar]
- 21.Myerson R, Emami B, Perez C, Straube W, Leybovich L, Von Gerichten D. Equilibrium temperature distributions in uniform phantoms for superficial microwave applicators: Implications for temperature based standards of applicator adequacy. Int J Hyperthermia. 1992;8:11–21. doi: 10.3109/02656739209052875. [DOI] [PubMed] [Google Scholar]
- 22.Rubin P, Constine LS, Fajardo LF, Phillips TL, Wasserman TH. RTOG Late Effects Working Group. Overview. Late Effects of Normal Tissues (LENT) scoring system. Int J Radiat Oncol Biol Phys. 1995;31:1041–1042. doi: 10.1016/0360-3016(95)00057-6. [DOI] [PubMed] [Google Scholar]
- 23.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 24.Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603–609. doi: 10.1016/0360-3016(86)90069-6. [DOI] [PubMed] [Google Scholar]
- 25.Turesson I. The progression rate of late radiation effects in normal tissue and its impact on dose–response relationships. Radiother Oncol. 1989;15:217–226. doi: 10.1016/0167-8140(89)90089-3. [DOI] [PubMed] [Google Scholar]