Abstract
Background
Gastrokine 1 (GKN1) acts as a gastric tumor suppressor. Here, we investigated whether GKN1 contributes to the maintenance of gastric mucosal homeostasis by regulating gastrin-induced gastric epithelial cell growth.
Methods
We assessed the effects of gastrin and GKN1 on cell proliferation in stable AGSGKN1 and MKN1GKN1 gastric cancer cell lines and HFE-145 nonneoplastic epithelial cells. Cell viability and proliferation were analyzed by MTT and BrdU incorporation assays, respectively. Cell cycle and expression of growth factor receptors were examined by flow cytometry and Western blot analyses.
Results
Gastrin treatment stimulated a significant time-dependent increase in cell viability and proliferation in AGSmock and MKN1mock, but not in HFE-145, AGSGKN1, and MKN1GKN1, cells, which stably expressed GKN1. Additionally, gastrin markedly increased the S-phase cell population, whereas GKN1 significantly inhibited the effect of gastrin by regulating the expression of G1/S cell-cycle regulators. Furthermore, gastrin induced activation of the NF-κB and β-catenin signaling pathways and increased the expression of CCKBR, EGFR, and c-Met in AGS and MKN1 cells. However, GKN1 completely suppressed these effects of gastrin via downregulation of gastrin/CCKBR/growth factor receptor expression. Moreover, GKN1 reduced gastrin and CCKBR mRNA expression in AGS and MKN1 cells, and there was an inverse correlation between GKN1 and gastrin, as well as between GKN1 and CCKBR mRNA expression in noncancerous gastric mucosae.
Conclusion
These data suggest that GKN1 may contribute to the maintenance of gastric epithelial homeostasis and inhibit gastric carcinogenesis by downregulating the gastrin-CCKBR signaling pathway.
Keywords: GKN1, Gastrin, Homeostasis, Cell growth, Stomach
Introduction
Gastrin is a gastrointestinal peptide hormone that has a key role in the regulation of gastric acid secretion and in gastric epithelial organization and maintenance by a variety of endocrine and paracrine mediators [1, 2]. Gastrin also regulates several important cellular processes in the gastric epithelium, including cell proliferation, apoptosis, migration, and invasion [3, 4]. Insulin-gastrin transgenic hypergastrinemic INS-GAS mice showed an increased proliferation of gastric epithelium, with dysplasia in 100 % and frank malignancy in 75 % of mice [5]. The observed malignant progression was significantly accelerated in Helicobacter felis-infected INS-GAS mice [5]. The infusion of gastrin at supraphysiological levels in humans was reported to result in increased gastric cell proliferation, as demonstrated by 3H-thymidine labeling studies [6]. Interestingly, hypergastrinemia associated with chronic Helicobacter pylori (H. pylori) infection may act as a co-factor during the development of gastric adenocarcinoma [4]. Taken together, these findings suggest that gastrin not only plays an important role in gastric tumorigenesis but can also be a potential therapeutic target for gastric cancer.
Gastrokine 1 (GKN1) encodes an 18-kDa antral mucosal protein (AMP-18) that is highly expressed in the antrum of the stomach [7]. GKN1 also protects gastric mucosa and promotes healing by facilitating restitution and proliferation after injury [7]. Interestingly, differential expression studies identified GKN1 as a gene strongly downregulated in H. pylori-infected gastric mucosal epithelial cells and gastric cancer [8–10], considering GKN1 as a putative stomach-specific tumor suppressor gene. Recently, it was shown that GKN1 induces senescence through the p16/Rb pathway activation in gastric cancer cells [11], and that its overexpression induces Fas-mediated apoptosis [12], suggesting that, in the absence of GKN1, gastric epithelial cells continuously proliferate without undergoing apoptosis. We previously reported that GKN1 has tumor suppressor activity through the regulation of epigenetic alterations and epithelial-mesenchymal transition [13, 14], implying its potential utility in clinical prediction/diagnosis. Thus, we hypothesized that GKN1 may contribute to the homeostasis of gastric mucosal epithelial cells by regulating gastrin activity.
In this study, we examined the impacts of GKN1 on gastrin activity in gastric cancer cell lines and gastric mucosal epithelium and demonstrated the effects of both genes on cell viability and proliferation, cell-cycle progression, and expression of growth factor receptors. Overall, we found that GKN1 may contribute to the homeostasis of gastric epithelial cells and suppress gastric carcinogenesis by inhibiting gastrin-induced cell proliferation.
Materials and methods
Cell culture and transfection of GKN1
AGS and MKN1 gastric cancer cell lines and HFE-145 nonneoplastic gastric epithelial cells were obtained from the American Type Culture Collection and Dr. Hassan (Washington, DC, USA). These cells were cultured at 37 °C in 5 % CO2 in RPMI-1640 medium (Lonza, Basel, Switzerland) supplemented with 10 % heat-inactivated fetal bovine serum. GKN1 cDNA was cloned into the pcDNA3.1 expression vector (Invitrogen, Carlsbad, CA, USA). We generated AGS and MKN1 cell lines, which stably expressed GKN1 (AGSGKN1 and MKN1GKN1 cells), as described previously [15]. Briefly, the human GKN1 expression vector was transfected into AGS and MKN1 cells using Lipofectamine 2000 (Invitrogen). The medium was changed after 24 h, and G418 (Wako, Osaka, Japan) was added to the culture medium to a final concentration of 1 mg/ml. Thereafter, cells were cultured in the presence of G418 for 8 weeks. The marked expression of GKN1 was confirmed by immunoblot analysis in HFE-145 cells and stable GKN1 transformants, AGSGKN1 and MKN1GKN1, but not in the stable mock cells, AGSmock and MKN1mock [15].
Measurement of cell viability and proliferation
We investigated whether the recombinant gastrin protein (Sigma, St. Louis, MO, USA) is involved in regulation of cell viability by an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] assay in AGSmock, MKN1mock, AGSGKN1, and MKN1GKN1 cells at 24, 48, and 72 h after treatment with gastrin (100 nM). MTT assay was also performed in HFE-145 cells after silencing of GKN1 by shGKN1 transfection, to further examine whether cell viability was dependent on activity of the GKN1 protein. Absorbance was measured with a spectrophotometer at 540 nm, and cell viability was expressed relative to the mock control.
For cell proliferation analysis, a BrdU incorporation assay was performed in AGSmock, MKN1mock, AGSGKN1, MKN1GKN1, and HFE-145 cells at 24, 48, and 72 h after treatment with gastrin (100 nM), using the BrdU cell proliferation assay kit (Millipore, Billerica, MA, USA) according to the manufacturer's instructions. Absorbance was measured with a spectrophotometer at 450 nm, and proliferation was expressed relative to the mock control.
Cell-cycle analysis by flow cytometry
To investigate the molecular mechanisms of gastrin-induced cell proliferation, gastrin (100 nM)-treated AGS and MKN1 cells were collected and stained with propidium iodide (PI) for 45 min in the dark before analysis. The percentages of cells in different phases of the cell cycle were determined using a FACSCalibur Flow Cytometer with CellQuest 3.0 software (BD Biosciences, Heidelberg, Germany). Experiments were performed in triplicate, and the average values were used for quantification.
Expression of cell-cycle regulators and growth factor receptors
We next determined whether the effect of gastrin on cell-cycle progression is blocked by GKN1. Expression of the G0/G1-phase proteins, including p53, p21, CDK6, cyclin D1, and β-catenin, was examined in AGSmock, MKN1mock, AGSGKN1, and MKN1GKN1 cells at 48 h after treatment with gastrin (100 nM). In addition, we analyzed the expression of gastrin receptor, cholecystokinin-B receptor (CCKBR), and growth factor receptors, such as epidermal growth factor receptor (EGFR) and c-Met, in AGS, MKN1, and HFE-145 cells at 48 h after treatment with gastrin (100 nM) and transfection with GKN1 or shGKN1. Cell lysates were separated on a 10 % polyacrylamide gel and transferred onto a Hybond PVDF membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA). After blocking, the membrane was subsequently probed with antibodies against target proteins. Protein bands were detected using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech).
The protein expression levels of the foregoing genes were assessed in c-myc-transfected and stable AGSGKN1 and MKN1GKN1 cells by Western blot analysis, to further investigate the mechanisms of GKN1-mediated expression of CCKBR and growth factor receptors. The following antibodies were used: CCKBR, GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA), c-myc, p-EGFR, and EGFR (Cell Signaling, Danvers, MA, USA). Additionally, the expression of gastrin and CCKBR mRNA transcripts were examined in c-myc-transfected and stable AGSGKN1 and MKN1GKN1 cells by real-time RT-PCR using SYBR Green Q-PCR Master Mix (Stratagene, La Jolla, CA, USA), according to the manufacturer's instructions. Each reaction was run for 40 cycles. To ensure the fidelity of mRNA extraction and reverse transcription, all samples were subjected to PCR amplification with oligonucleotide primers specific for the constitutively expressed gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and normalized. The primer sequences are listed in Table 1. All samples were tested in triplicate. Data are reported as relative quantities, according to an internal calibrator using the 2−ΔΔCT method [16].
Table 1.
Primer sequences for real-time RT-PCR
| Gene | Primer sequences |
|---|---|
| GKN1 | F: 5′-CAAAGTCGATGACCTGAGCA-3′ |
| R: 5′-CTTGCCTCTTGCATCTCCTC-3′ | |
| Gastrin | F: 5′-CTTAGGTACAGGGGCCAACA-3′ |
| R: 5′-TCCATCCATCCATAGGCTTC-3′ | |
| CCKBR | F: 5′-GCCTGAGGACTGTCACCAAT-3′ |
| R: 5′-ACCCCCATGAGGTAGGAAAC-3′ | |
| GAPDH | F: 5′-AAATCAAGTGGGGCGATGCTG-3′ |
| R: 5′-GCAGAGATGATGACCCTTTTG-3′ |
Expression of NF-κB pathway proteins
To determine whether gastrin is involved in regulation of the NF-κB signaling pathway, the expression of NF-κB-related proteins, including NF-κB p-p65, NF-κB p65, IKKα/β, and IκB, was examined in mock and GKN1 stable AGS and MKN1 cells at 48 h after treatment with gastrin (100 nM). Cell lysates were separated on a 10 % polyacrylamide gel and transferred onto a Hybond PVDF membrane (Amersham Pharmacia Biotech). After blocking, the membrane was subsequently probed with antibodies against NF-κB p-p65, NF-κB p65, IKKα/β, and IκB (Cell Signaling). Protein bands were detected using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech).
Expression of GKN1, gastrin, and CCKBR in gastric cancer cell lines and gastric mucosae
Expression of gastrin and CCKBR mRNA transcripts was analyzed in stable AGSGKN1 and MKN1GKN1 cells by real-time RT-PCR. Also, the expression of gastrin and CCKBR was examined in 55 frozen noncancerous gastric mucosae by real-time RT-PCR and compared with the expression level of GKN1. Real-time RT-PCR was performed using SYBR Green Q-PCR Master Mix (Stratagene), according to the manufacturer's instructions. Each reaction was run for 40 cycles. Gastrin, CCKBR, and GKN1 mRNAs were quantified by SYBR Green Q-PCR and normalized to mRNA of the housekeeping gene, GAPDH. The primer sequences are described in Table 1. The standard curve method was used for quantification of the relative amounts of gene expression products. This method provides unit-less normalized expression values that can be used for direct comparison of the relative amount of mRNA in different samples. All samples were tested in triplicate. Data are reported as relative quantities, according to an internal calibrator using the 2−ΔΔCT method [16]. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board of The Catholic University of Korea, College of Medicine (CUMC09U089).
Statistical analysis
The effects of gastrin and GKN1 on cell viability, proliferation, and cell-cycle progression were assessed by Student's t test. Data are expressed as mean values ± SD from at least three independent experiments. Spearman's correlation test was used to investigate relationships between GKN1, gastrin, and CCKBR mRNA expression in 55 noncancerous gastric mucosae. Statistical analyses were performed using Graphpad 5.0 (GraphPad software, San Diego, CA, USA). A P value less than 0.05 was considered statistically significant.
Results
GKN1 inhibits gastrin-induced cell growth
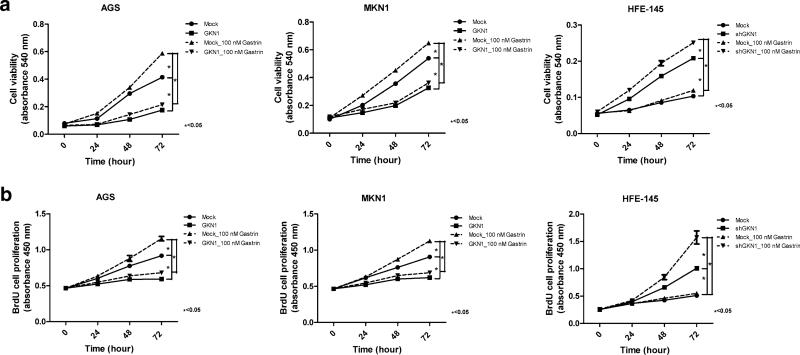
Stable transfectants of AGS and MKN1 cells that stably expressed the GKN1 protein (AGSGKN1 and MKN1GKN1 cells) were generated to investigate whether GKN1 inhibits gastrin-induced cell growth. AGSGKN1 and MKN1GKN1 cells, but not AGSmock and MKN1mock cells, showed marked expression of the GKN1 protein [15]. There was a significant time-dependent increase in cell viability in AGS and MKN1 cells treated with gastrin, compared to untreated mock cells, whereas gastrin treatment had no effect on cell viability in HFE-145, AGSGKN1, and MKN1GKN1 cells, which stably expressed the GKN1 protein (P < 0.05) (Fig. 1a). Silencing of GKN1 with shGKN1 in HFE-145 cells resulted in a gastrin-induced time-dependent increase in cell viability (P < 0.05) (Fig. 1a).
Fig. 1.
Gastrokine 1 (GKN1) inhibits gastrin-induced cell growth. a In the MTT assay, AGS and MKN1 cells treated with gastrin (100 nM) exhibited a significant increase in cell viability in a time-dependent manner, whereas no effect of gastrin on cell viability was found in HFE-145, AGSGKN1, and MKN1GKN1 cells, which stably expressed the GKN1 protein (P < 0.05). In HFE-145 cells, GKN1 silencing by transfection with shGKN1 led to gastrin-induced time-dependent increase in cell viability (P < 0.05). b In the BrdU incorporation assay, gastrin treatment resulted in a time-dependent augmentation of cell proliferation in AGSmock and MKN1mock cells, but not in HFE-145 and stable AGSGKN1 and MKN1GKN1 cells (P < 0.05). When GKN1 was silenced by specific shGKN1 in HFE-145 cells, gastrin treatment facilitated cell proliferation in a time-dependent manner (P < 0.05). Data are expressed as mean values ± SD from at least three independent experiments. Unpaired Student's t test was used to analyze statistical difference in cell viability and proliferation between experimental groups. P < 0.05 was considered statistically significant
In the BrdU incorporation assay, treatment with gastrin also led to a time-dependent augmentation of cell proliferation in AGSmock and MKN1mock cells, but not in HFE-145 and GKN1 stable AGSGKN1 and MKN1GKN1 cells (P < 0.05) (Fig. 1b). When GKN1 was silenced by specific shGKN1 in HFE-145 cells, gastrin treatment enhanced cell proliferation in a time-dependent manner (P < 0.05) (Fig. 1b).
GKN1 inhibits gastrin-induced cell-cycle progression
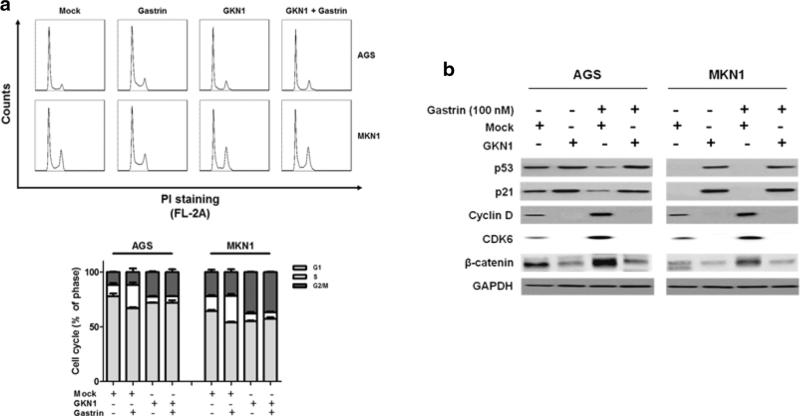
Gastrin treatment stimulated a considerable increase in the S-phase cell population (P = 0.038 and 0.014 in AGS and MKN1 cells, respectively), whereas stable expression of GKN1 in AGSGKN1 and MKN1GKN1 cells significantly inhibited the effect of gastrin on cell-cycle progression in flow cytometry analysis (P = 0.012 and 0.005, respectively) (Fig. 2a). Interestingly, GKN1 stable cells, in contrast to gastrin-treated AGS and MKN1 cells, also exhibited a modest effect on G2/M-phase cell-cycle progression (P = 0.002 and 0.01, respectively) (Fig. 2a). We therefore examined the expression of G0/G1-phase-related proteins by Western blot. In AGSmock and MKN1mock cells, gastrin increased the expression of cyclin D1 and Cdk6, whereas GKN1 upregulated the expression of p53 and p21 and downregulated the expression of Cdk6 and cyclin D1 in AGSGKN1 and MKN1GKN1 cells (Fig. 2b). In addition, gastrin treatment increased the expression of β-catenin in AGSmock and MKN1mock cells, whereas GKN1 markedly downregulated its expression, even in gastrin-treated AGSGKN1 and MKN1GKN1 cells (Fig. 2b).
Fig. 2.
GKN1 inhibits gastrin-induced cell-cycle progression. a Treatment with gastrin considerably increased the percentage of cells in S-phase (P = 0.038 and 0.014 in AGS and MKN1 cells, respectively), whereas the stable expression of GKN1 in AGSGKN1 and MKN1GKN1 cells significantly restrained this effect of gastrin on cell-cycle progression (P = 0.012 and 0.005, respectively). GKN1 stable cells also showed a modest effect on G2/M-phase cell-cycle progression in AGS and MKN1 cells (P = 0.002 and 0.01, respectively), although gastrin did not (P > 0.05). The experiments were performed in triplicate, and the average values were used for quantification. Unpaired Student's t test was used to analyze statistical difference between experimental groups. P < 0.05 was considered statistically significant. b Gastrin enhanced the expression of cyclin D1, Cdk6, and β-catenin. However, GKN1 upregulated the expression of p53 and p21 and downregulated the expression of cyclin D1, Cdk6, and β-catenin in AGSGKN1 and MKN1GKN1 cells, even after treatment with gastrin. Data shown are representative of at least three independent experiments
GKN1 downregulates the expression of CCKBR and growth factor receptors
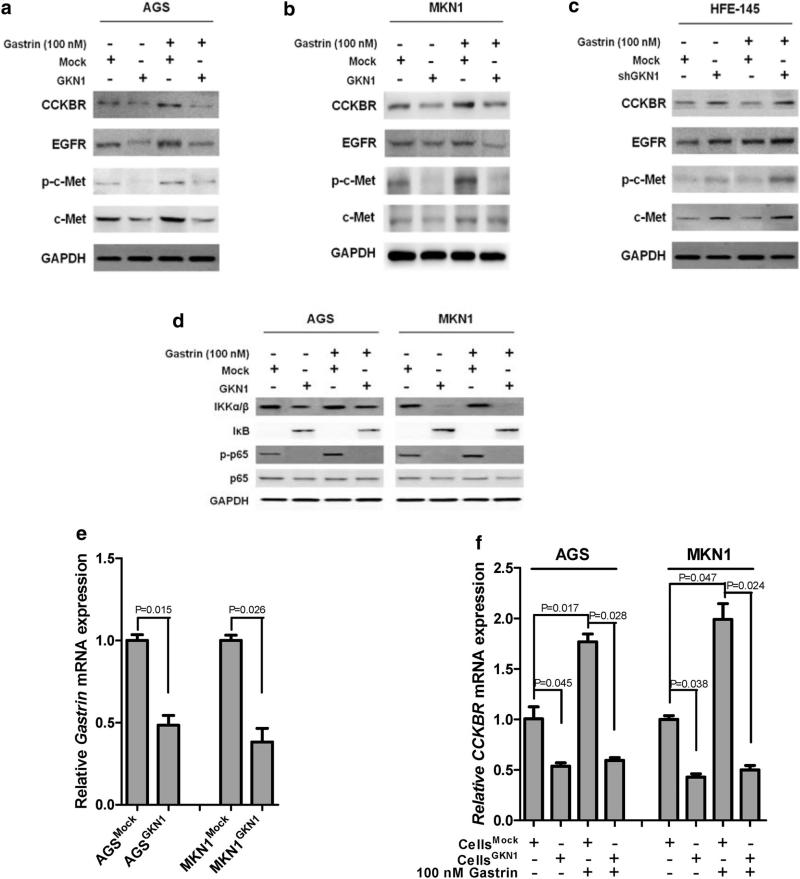
To verify the inhibitory effect of GKN1 on gastrin-induced cell-cycle progression, we examined whether GKN1 regulates the expression of CCKBR, which is a receptor for gastrin, and growth factor receptors, including EGFR and c-Met. Interestingly, gastrin promoted increased expression of CCKBR, EGFR, p-c-Met, and c-Met in AGS and MKN1 cells (Fig. 3a, b). However, significantly decreased levels of these proteins were detected in the AGS cells transfected with GKN1, despite treatment with gastrin (Fig. 3a, b), whereas GKN1 silencing in HFE-145 cells resulted in increased expression of CCKBR, EGFR, and c-Met proteins (Fig. 3c).
Fig. 3.
GKN1 downregulates gastrin-induced expression of CCKBR, growth factor receptors, and NF-κB-related proteins. a, b Gastrin increased the expression of CCKBR, EGFR, p-c-Met, and c-Met in AGS and MKN1 cells, whereas GKN1 significantly decreased the expression levels of these proteins, even in the cells treated with gastrin. c GKN1 silencing in HFE-145 cells resulted in elevated expression of the CCKBR, EGFR, and c-Met proteins. d Gastrin enhanced the expression of p-p65 and slightly increased that of IKKα/β, although it did not affect the expression of p65 and IκB proteins. However, GKN1 reverted the expression of IκB and decreased the expression of IKKα/β, p-p65, and p65 proteins. Data shown are representative of at least three independent experiments. e A significant decline in mRNA expression of gastrin was observed in AGS and MKN1 cells stably expressing the GKN1 protein (P = 0.015 and P = 0.026, respectively). f Gastrin markedly enhanced CCKBR mRNA expression in AGS and MKN1 cells (P = 0.017 and P = 0.047, respectively), whereas GKN1 completely suppressed the increase in the CCKBR mRNA levels induced by gastrin in both cell lines (P = 0.028 and P = 0.024, respectively). Experiments were performed in triplicate. Data are reported as relative quantities, according to an internal calibrator using the 2−ΔΔCT method [16]. A Student's t test (P < 0.05) was used to analyze the statistical difference in Gastrin or CCKBR mRNA expression between experimental groups
GKN1 inhibits gastrin-induced activation of the NF-κB signaling pathway
Because treatment with gastrin is reported to result in activation of the NF-κB signaling pathway [17], we next investigated whether the NF-κB pathway is activated in response to gastrin via the canonical pathway involving IKKα/β and IκB in AGS and MKN1 cells. Gastrin enhanced the expression of p-p65 and slightly increased that of IKKα/β, although it did not affect the expression of p65 and IκB proteins. However, GKN1 reverted the expression of IκB and decreased the expression of IKKα/β, p-p65, and p65 proteins (Fig. 3d).
We next analyzed the mRNA expression of gastrin and CCKBR in AGSmock, MKN1mock, AGSGKN1, and MKN1GKN1 cells. Both gastrin and CCKBR mRNA levels were significantly diminished in AGS and MKN1 cells stably expressing the GKN1 protein (P = 0.015 and P = 0.026; P = 0.045 and P = 0.038; respectively) (Fig. 3e, f). Expectedly, CCKBR mRNA expression was markedly elevated in AGS and MKN1 cells treated with gastrin (P = 0.017 and P = 0.047, respectively) (Fig. 3f). Nevertheless, GKN1 totally inhibited gastrin-induced expression of CCKBR mRNA in AGS and MKN1 cells (P = 0.028 and P = 0.024, respectively) (Fig. 3f).
GKN1 inhibits the c-myc-induced expression of CCKBR
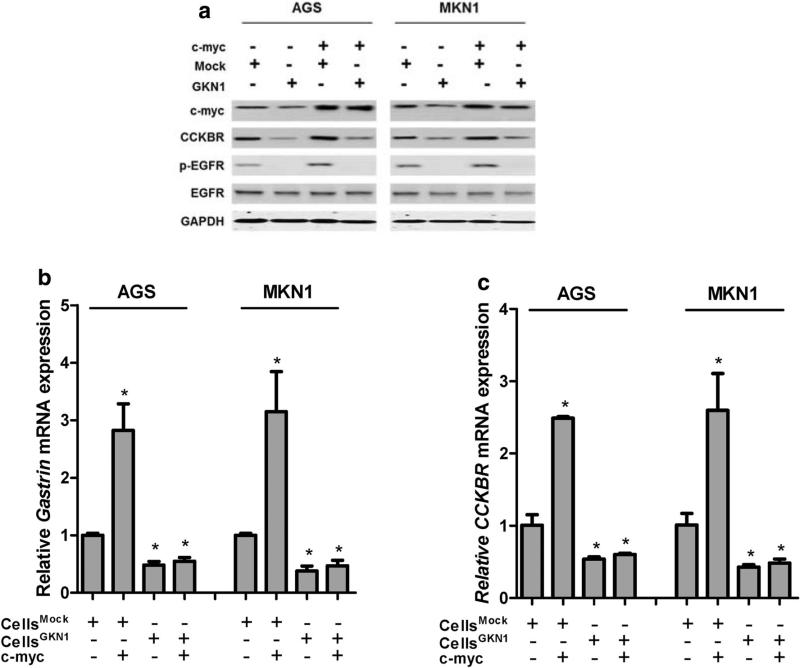
To determine the molecular mechanism of the GKN1-induced downregulation of CCKBR, we assessed CCKBR protein expression in AGSGKN1 and MKN1GKN1 cells after transient transfection with c-myc. The ectopic expression of c-myc markedly increased the CCKBR expression in AGS and MKN1 cells, whereas GKN1 completely suppressed the effect of c-myc on the CCKBR gene in both cell lines (Fig. 4a). In addition, GKN1 inhibited the expression of p-EGFR in AGS and MKN1 cells, even in those transfected with c-myc (Fig. 4a).
Fig. 4.
GKN1 inhibits the c-myc-induced expression of CCKBR. a The ectopic expression of c-myc markedly increased the expression of CCKBR and p-EGFR in AGS and MKN1 cells, whereas GKN1 counteracted the effects of c-myc in both cell lines. Data shown are representative of at least three independent experiments. b, c mRNA levels of gastin and CCKBR were dramatically augmented in c-myc-transfected AGS and MKN1 cells (P = 0.029 and 0.045, respectively). However, GKN1 significantly downregulated gastrin and CCKBR mRNA expression (P = 0.008 and 0.01) and abrogated the stimulating effect of c-myc on both genes (P = 0.02 and 0.031, respectively). Experiments were performed in triplicate. Data are reported as relative quantities, according to an internal calibrator using the 2−ΔΔCT method [16]. Unpaired Student's t test (P < 0.05) was used to analyze statistical difference in Gastrin or CCKBR mRNA expression between experimental groups
Similarly, gastrin and CCKBR mRNA levels were dramatically augmented in c-myc-transfected AGS and MKN1 cells (P = 0.029 and 0.045, respectively) (Fig. 4b, c). However, GKN1 significantly downregulated the mRNA expression of gastrin and CCKBR (P = 0.008 and 0.01) and abrogated the stimulating effect of c-myc on both genes (P = 0.02 and 0.031, respectively) (Fig. 4b, c).
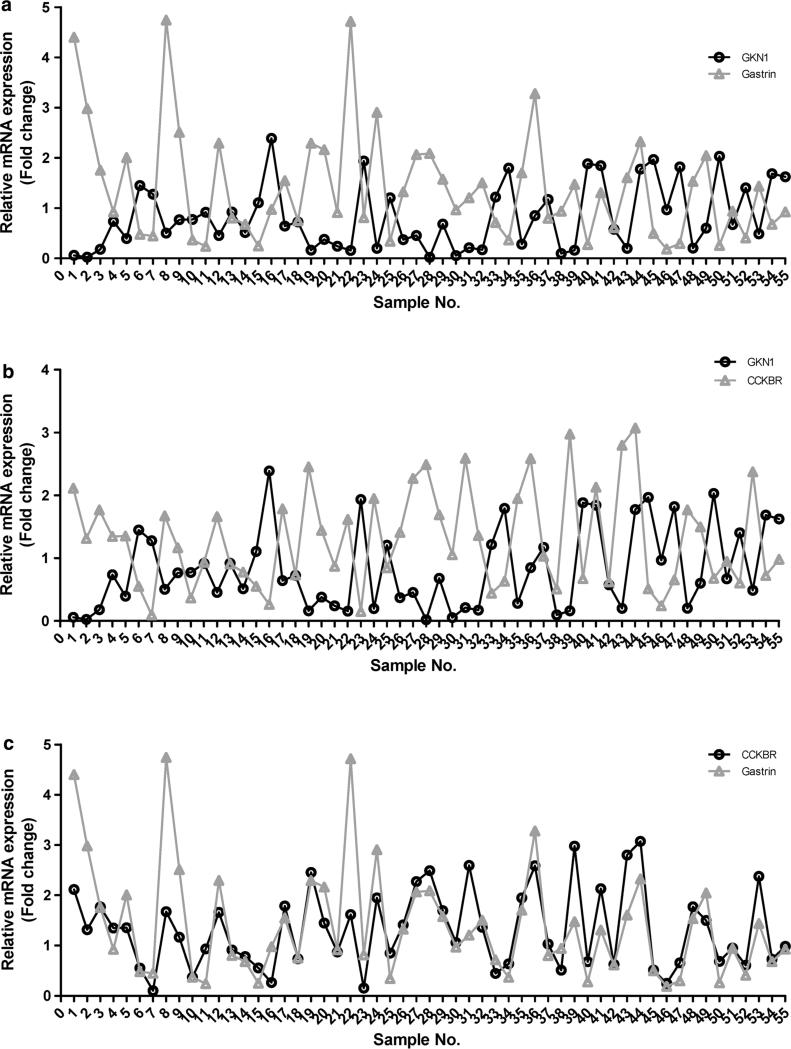
GKN1 inversely correlates with the gastrin/CCKBR mRNA expression in noncancerous gastric mucosae
To confirm our previous observations, we also analyzed the expression of GKN1, CCKBR, and gastrin mRNA transcripts in 55 noncancerous gastric mucosae by real-time RT-PCR and found an inverse correlation in the mRNA transcript expression between the GKN1 and gastrin genes (P < 0.0001) (Fig. 5a). Moreover, an inverse correlation between GKN1 and CCKBR was also observed (P < 0.0001) (Fig. 5b). Expectedly, gastrin mRNA expression exhibited a positive correlation with that of CCKBR (P < 0.0001), as shown in Fig. 5c.
Fig. 5.
GKN1 inhibits the gastin/CCKBR mRNA expression in gastric mucosae. a The expression of GKN1, CCKBR, and gastrin mRNA transcripts was analyzed in 55 noncancerous gastric mucosae by real-time RT-PCR. There was an inverse correlation in mRNA transcript expression between the GKN1 and gastrin genes (Spearman's correlation test, P < 0.0001). b GKN1 and CCKBR mRNA expression levels were inversely correlated in noncancerous gastric mucosae (Spearman's correlation test, P < 0.0001). c Gastrin mRNA expression exhibited a positive correlation with that of CCKBR (Spearman's correlation test, P < 0.0001)
Discussion
Generally, gastrointestinal epithelium is characterized by a very high cellular turnover rate, which leads to epithelial renewal every 3 to 5 days, and apoptosis is a key regulator of this turnover [18]. Continuous processes of cell proliferation, differentiation, and self-renewal are counterbalanced by apoptosis, thus maintaining gastric epithelial homeostasis. A large body of evidence showed that gastrin, acting through the cholecystokinin-B receptor (CCKBR), plays a significant role in the proliferation of gastric epithelial cells and may be abnormally expressed in gastrointestinal carcinoma cells [19, 20]. It was reported that gastrin and CCKBR are co-expressed in human gastric carcinoma tissues [21] and that the exogenous gastrin exhibits a potent stimulatory effect on the proliferation of gastric cancer cells [22, 23]. In the present study, we similarly demonstrated that gastrin stimulates growth of gastric cancer cells (P < 0.05) (Fig. 1a, b). However, gastrin-induced cell growth was dramatically inhibited in GKN1 stably transfected AGS and MKN1 cells, and in HFE-145 cells expressing GKN1 (P < 0.05) (Fig. 1a, b). Additionally, treatment of HFE-145 cells with gastrin after GKN1 silencing by transfection with shGKN1 significantly enhanced cell proliferation (P < 0.05) (Fig. 1a, b). Thus, these data suggest that GKN1 may contribute to gastric mucosal homeostasis and gastric carcinogenesis by regulating gastrin-induced cell proliferation.
Previous studies have demonstrated that gastrin promotes cell proliferation as well as increasing the proportion of the S-phase cell population by upregulating cyclin D1 and CDK4 via activation of the β-catenin and CRE-binding protein pathways [24–26]. The fact that GKN1 suppresses gastrin-induced cell proliferation implies that GKN1 can modulate cell-cycle progression by regulating gastrin. We found a concomitant increase of the S-phase cell population in gastrin-treated AGS and MKN1 cells (P = 0.038 and 0.014, respectively), whereas no effect of gastrin on the G2/M-phase of the cell cycle was detected in flow cytometry analysis (Fig. 2a). Interestingly, GKN1 completely reverted the increase of the S-phase population in gastrin-treated AGS and MKN1 cells (P = 0.012 and 0.005, respectively) and induced G2/M arrest (P = 0.002 and 0.01, respectively) (Fig. 2a). These results suggest that GKN1 may inhibit gastrin-induced cell-cycle progression and corroborate the previous observation that GKN1 induces G0/G1 and G2/M arrests [13]. In addition, gastrin treatment increased the expression of positive cell-cycle regulators, including cyclin D1, Cdk6, and β-catenin, whereas GKN1 suppressed the expression of these proteins and upregulated the expression of negative cell-cycle regulators, such as p53 and p21 (Fig. 2b). These findings suggest that GKN1 counteracts the proliferative effects of gastrin by modulating the expression of cell-cycle regulators, especially those related to the G1/S-phase transition.
Binding of gastrin to CCKBR in gastric epithelial cells induced the expression and release of heparin-binding epidermal growth factor-like growth factor, which subsequently transactivated epithermal growth factor receptor (EGFR) and its downstream signaling pathways [27, 28]. Also, it was reported that increase of epidermal growth factor (EGF) and EGFR expression was found in the gastric mucosae of patients with chronic gastritis and gastric cancer [29, 30]. In addition, the expression of gastrin and c-Met proteins was significantly increased in gastric cancer [31]. Furthermore, gastrin markedly enhanced the endogenous expression of CCKBR, and overexpression of CCKBR protein was detected in the stomach of hypergastrinaemic animals [32]. Here, to further clarify the mechanism underlying the inhibitory activity of GKN1 on gastrin-induced cell proliferation, we set out to determine if GKN1 inhibits the expression of the gastrin-specific receptor, CCKBR, and growth factor receptors. Gastrin treatment stimulated the increased expression of CCKBR, EGFR, and c-Met proteins, whereas GKN1 completely abrogated the expression of these genes in AGS and MKN1 cells (Fig. 3a, b). Conversely, GKN1 silencing with shGKN1 increased the expression of CCKBR, EGFR, and c-Met proteins in HFE-145 cells (Fig. 3c). Additionally, as it was previously reported that gastrin is capable of activating the NF-κB signaling pathway [17], we investigated whether the NF-κB pathway is activated in response to gastrin via the canonical pathway, involving IKKα/β and IκB, in AGS and MKN1 cells. Gastrin enhanced the expression of p-p65 and slightly increased IKKα/β, although it did not affect the expression of p65 and IκB proteins. However, GKN1 reverted the expression of IκB and inactivated the expression of IKKα/β, p-p65, and p65 proteins (Fig. 3d). Interestingly, GKN1 reduced the mRNA expression of both gastrin and CCKBR in AGS and MKN1 gastric cancer cells (P = 0.015 and P = 0.026; P = 0.045 and P = 0.038, respectively) (Fig. 3e, f). These results indicate that GKN1 may inhibit cell proliferation through suppression of the gastrin-CCKBR-growth factor receptor and NF-κB signaling pathways, thus contributing to gastric mucosal homeostasis.
It has been reported that gastrin activates β-catenin/Tcf-4 signaling and thereby induces the expression of CCKBR and early responsive genes, such as c-myc [32–34]. Previously, we found that GKN1 directly binds to the c-myc protein and downregulates its expression in immunoprecipitation assay [15]. In this study, we examined whether GKN1 downregulates CCKBR by inactivating the c-myc-induced gastrin expression. A significantly increased level of the CCKBR protein was detected in AGSmock and MKN1mock cells transfected with c-myc, but the c-myc transfection had no effect on the CCKBR expression in both stable cell lines expressing GKN1 (Fig. 4a). Interestingly, the mRNA levels of gastrin and CCKBR were dramatically increased in c-myc-transfected AGS and MKN1 cells (P = 0.029 and 0.045, respectively), whereas GKN1 significantly diminished the mRNA expression of both genes (P = 0.008 and 0.01), even in cells transfected with c-myc (P = 0.02 and 0.031, respectively) (Fig. 4b, c). These observations suggest that GKN1 may inhibit the expression of CCKBR by downregulating c-myc activity.
It is well known that the main site of gastrin synthesis is the G cell within the antropyloric mucosa [20, 35], whereas the GKN1 protein and mRNA de novo synthesis is confirmed in surface mucous cells of the gastric antrum and fundus in the human [10, 36–38], mouse [36], and chicken [39]. In this study, we examined 55 noncancerous gastric mucosa samples, and an inverse relationship between gastrin and GKN1 (P < 0.0001) (Fig. 5a), as well as between CCKBR and GKN1 mRNA expression (P < 0.0001) (Fig. 5b), was found. Additionally, a positive association between gastrin and CCKBR mRNA transcripts was observed (P < 0.0001) (Fig. 5c). These findings imply that the physiological synthesis of gastrin in G cells and its absence in gastric epithelial cells may be maintained by the abundant expression of GKN1, which inhibits the gastrin-induced cell proliferation and thereby is important in gastric mucosal homeostasis-preserving mechanisms.
In conclusion, the present study demonstrates a novel function of a gastric tumor suppressor gene, GKN1, in maintaining gastric mucosal homeostasis and inhibiting gastric carcinogenesis, arising through repression of gastrin-induced cell proliferation. Gastrin stimulated a significant time-dependant augmentation of gastric cancer cell viability and proliferation as well as inducing cell-cycle progression by increasing the S-phase cell population. Furthermore, gastrin facilitated the expression of CCKBR and growth factor receptors, including EGFR and c-Met, and activated the NF-κB signaling pathway in AGS and MKN1 gastric cancer cells. However, GKN1 completely restrained these impacts of gastrin on gastric cancer cell proliferation by downregulating the gastrin-CCKBR-growth factor receptor and NF-κB signaling pathways. Moreover, analysis of 55 noncancerous gastric mucosa samples showed an inverse relationship between GKN1 and gastrin, as well as between GKN1 and CCKBR mRNA expression. Expectedly, a positive association between gastrin and CCKBR mRNA transcripts was observed. Although the exact molecular mechanisms regulating the growth-promoting effect of gastrin remain to be fully elucidated, this is the first report that GKN1 may suppress gastrin-induced cell proliferation by downregulating the gastrin-CCKBR-growth factor receptor and NF-κB signaling pathways and thereby contribute to gastric mucosal homeostasis and gastric carcinogenesis. Additional functional and translational studies of GKN1 and gastrin will broaden our understanding of the physiological homeostasis of gastric mucosal cells and provide us with novel diagnostic and therapeutic modalities for preventing gastric cancer.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A2A2A01002531).
Footnotes
Conflict of interest The authors disclose no potential conflicts of interest.
Contributor Information
Olga Kim, Department of Pathology, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Seocho-gu, Seoul 137-701, Korea.
Jung Hwan Yoon, Department of Pathology, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Seocho-gu, Seoul 137-701, Korea.
Won Suk Choi, Department of Pathology, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Seocho-gu, Seoul 137-701, Korea.
Hassan Ashktorab, Department of Medicine, Howard University, Washington, DC 20060, USA.
Duane T. Smoot, Department of Medicine, Howard University, Washington, DC 20060, USA
Suk Woo Nam, Department of Pathology, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Seocho-gu, Seoul 137-701, Korea.
Jung Young Lee, Department of Pathology, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Seocho-gu, Seoul 137-701, Korea.
Won Sang Park, Department of Pathology, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Seocho-gu, Seoul 137-701, Korea.
References
- 1.Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–39. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 2.Edkins JS. The chemical mechanism of gastric secretion. J Physiol. 1906;34(1-2):133–44. doi: 10.1113/jphysiol.1906.sp001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishizuka J, Marinez J, Townsend DM, Jr, Thompson JC. The effect of gastrin on growth of human stomach cancer cells. Ann Surg. 1992;215(5):528–34. doi: 10.1097/00000658-199205000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkitt MD, Varro A, Pritchard DM. Importance of gastrin in the pathogenesis and treatment of gastric tumors. World J Gastroenterol. 2009;15(1):1–16. doi: 10.3748/wjg.15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118(1):36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 6.Hansen OH, Pedersen T, Larsen JK, Rehfeld JF. Effect of gastrin on gastric mucosal cell proliferation in man. Gut. 1976;17(7):536–41. doi: 10.1136/gut.17.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toback FG, Walsh-Reitz MM, Musch MW, Chang EB, Del Valle J, Ren H, et al. Peptide fragments of AMP-18, a novel secreted gastric antrum mucosal protein, are mitogenic and motogenic. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G344–53. doi: 10.1152/ajpgi.00455.2002. [DOI] [PubMed] [Google Scholar]
- 8.Shiozaki K, Nakamori S, Tsujie M, Okami J, Yamamoto H, Nagano H, et al. Human stomach-specific gene, CA11, is down-regulated in gastric cancer. Int J Oncol. 2001;19(4):701–7. doi: 10.3892/ijo.19.4.701. [DOI] [PubMed] [Google Scholar]
- 9.Oien KA, McGregor F, Butler S, Ferrier RK, Downie I, Bryce S, et al. Gastrokine 1 is abundantly and specifically expressed in superficial gastric epithelium, down-regulated in gastric carcinoma, and shows high evolutionary conservation. J Pathol. 2004;203(3):789–97. doi: 10.1002/path.1583. [DOI] [PubMed] [Google Scholar]
- 10.Nardone G, Rippa E, Martin G, Rocco A, Siciliano RA, Fiengo A, et al. Gastrokine 1 expression in patients with and without Helicobacter pylori infection. Dig Liver Dis. 2007;39(2):122–9. doi: 10.1016/j.dld.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Xing R, Li W, Cui J, Zhang J, Kang B, Wang Y, et al. Gastrokine 1 induces senescence through p16/Rb pathway activation in gastric cancer cells. Gut. 2012;64(1):43–52. doi: 10.1136/gut.2010.230623. [DOI] [PubMed] [Google Scholar]
- 12.Rippa E, La Monica G, Allocca R, Romano MF, De Palma M, Arcari P. Overexpression of gastrokine 1 in gastric cancer cells induces Fas-mediated apoptosis. J Cell Physiol. 2011;226(10):2571–8. doi: 10.1002/jcp.22601. [DOI] [PubMed] [Google Scholar]
- 13.Yoon JH, Choi YJ, Choi WS, Ashktorab H, Smoot DT, Nam SW, et al. GKN1-miR-185-DNMT1 axis suppresses gastric carcino-genesis through regulation of epigenetic alteration and cell cycle. Clin Cancer Res. 2013;19(17):4599–610. doi: 10.1158/1078-0432.CCR-12-3675. [DOI] [PubMed] [Google Scholar]
- 14.Yoon JH, Kang YH, Choi YJ, Park IS, Nam SW, Lee JY, et al. Gastrokine 1 functions as a tumor suppressor by inhibition of epithelial-mesenchymal transition in gastric cancers. J Cancer Res Clin Oncol. 2011;137(11):1697–704. doi: 10.1007/s00432-011-1051-8. [DOI] [PubMed] [Google Scholar]
- 15.Yoon JH, Seo HS, Choi WS, Kim O, Nam SW, Lee JY, et al. Gastrokine 1 induces senescence and apoptosis through regulating telomere length in gastric cancer. Oncotarget. 2014;5(22):11695–708. doi: 10.18632/oncotarget.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogasa M, Miyazaki Y, Hiraoka S, Kitamura S, Nagasawa Y, Kishida O, et al. Gastrin activates nuclear factor jB (NFjB) through a protein kinase C dependent pathway involving NFjB inducing kinase, inhibitor jB (IjB) kinase, and tumour necrosis factor receptor associated factor 6 (TRAF6) in MKN-28 cells transfected with gastrin receptor. Gut. 2003;52(6):813–9. doi: 10.1136/gut.52.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(pt 12):3569–77. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 19.Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochim Biophys Acta. 2004;1704(1):1–10. doi: 10.1016/j.bbcan.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Watson SA, Brabowska AM, El-Zaatarim M, Takhar A. Gastrin-active participant or bystander in gastric carcinogenesis? Nat Rev Cancer. 2006;6(12):936–46. doi: 10.1038/nrc2014. [DOI] [PubMed] [Google Scholar]
- 21.Zhou JJ, Chen ML, Zhang QZ, Hu JK, Wang WL. Coexpression of cholecystokinin-B/gastrin receptor and gastrin gene in human gastric tissues and gastric cancer cell line. World J Gastroenterol. 2004;10(6):791–4. doi: 10.3748/wjg.v10.i6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song DH, Rana B, Wolfe JR, Crimmins G, Choi C, Albanese C, et al. Gastrin-induced gastric adenocarcinoma growth is mediated through cyclin D1. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G217–22. doi: 10.1152/ajpgi.00516.2002. [DOI] [PubMed] [Google Scholar]
- 23.Xu W, Chen GS, Shao Y, Li XL, Xu HC, Zhang H, et al. Gastrin acting on the cholecystokinin 2 receptor induces cyclooxygenase-2 expression through JAK2/STAT3/PI3K/Akt pathway in human gastric cancer cells. Cancer Lett. 2013;332(1):11–8. doi: 10.1016/j.canlet.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Koh TJ, Bulitta CJ, Fleming JV, Dockray GJ, Varro A, Wang TC. Gastrin is a target of the beta-catenin/TCF-4 growth-signaling pathway in a model of intestinal polyposis. J Clin Invest. 2000;106(4):533–9. doi: 10.1172/JCI9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pradeep A, Sharma C, Sathyanarayana P, Albanese C, Fleming JV, Wang TC, et al. Gastrin-mediated activation of cyclin D1 transcription involves beta-catenin and CREB pathways in gastric cancer cells. Oncogene. 2004;23(20):3689–99. doi: 10.1038/sj.onc.1207454. [DOI] [PubMed] [Google Scholar]
- 26.Han YM, Park JM, Park SH, Hahm KB, Hong SP, Kim EH. Gastrin promotes intestinal polyposis through cholecystokinin-B receptor-mediated proliferative signaling and fostering tumor microenvironment. J Physiol Pharmacol. 2013;64(4):429–37. [PubMed] [Google Scholar]
- 27.Noble PJ, Wilde G, White MR, Pennington SR, Dockray GJ, Varro A. Stimulation of gastrin-CCKB receptor promotes migration of gastric AGS cells via multiple paracrine pathways. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G75–84. doi: 10.1152/ajpgi.00300.2002. [DOI] [PubMed] [Google Scholar]
- 28.Sinclair NF, Ai W, Raychowdhury R, Bi M, Wang TC, Koh TJ, et al. Gastrin regulates the heparin-binding epidermal-like growth factor promoter via a PKC/EGFR-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2004;286(6):G992–9. doi: 10.1152/ajpgi.00206.2002. [DOI] [PubMed] [Google Scholar]
- 29.Slesak B, Harlozinska A, Porebska I, Bojarowski T, Lapinska J, Rzeszutko M, et al. Expression of epidermal growth factor receptor family proteins (EGFR, c-erbB-2 and c-erbB-3) in gastric cancer and chronic gastritis. Anticancer Res. 1998;18(4A):2727–32. [PubMed] [Google Scholar]
- 30.Jurkowska G, Piotrowska-Staworko G, Guzińska-Ustymowicz K, Kemona A, Swidnicka-Siergiejko A, Laszewicz W, et al. The impact of Helicobacter pylori on EGF, EGF receptor, and the c-erb-B2 expression. Adv Med Sci. 2014;59(2):221–6. doi: 10.1016/j.advms.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Tang Z, Zhao M, Ji J, Yang G, Hu F, He J, et al. Overexpression of gastrin and c-met protein involved in human gastric carcinomas and intestinal metaplasia. Oncol Rep. 2004;11(2):333–9. [PubMed] [Google Scholar]
- 32.Ashurst HL, Varro A, Dimaline R. Regulation of mammalian gastrin/CCK receptor (CCK2R) expression in vitro and in vivo. Exp Physiol. 2008;93(2):223–36. doi: 10.1113/expphysiol.2007.040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taniguchi T, Matsui T, Ito M, Murayama T, Tsukamoto T, Katakami Y, et al. Cholecystokinin-B/gastrin receptor signaling pathway involves tyrosine phosphorylations of p125FAK and p42MAP. Oncogene. 1994;9(3):861–7. [PubMed] [Google Scholar]
- 34.Cao J, Yu JP, Liu CH, Zhou L, Yu HG. Effects of gastrin 17 on beta-catenin/Tcf-4 pathway in Colo320WT colon cancer cells. World J Gastroenterol. 2006;12(46):7482–7. doi: 10.3748/wjg.v12.i46.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd KC. Gut hormones in gastric function. Baillieres Clin Endocrinol Metab. 1994;8(1):111–36. doi: 10.1016/s0950-351x(05)80228-9. [DOI] [PubMed] [Google Scholar]
- 36.Martin TE, Powell CT, Wang Z, Bhattacharyya S, Walsh-Reitz MM, Agarwal K, et al. A novel mitogenic protein that is highly expressed in cells of the gastric antrum mucosa. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G332–43. doi: 10.1152/ajpgi.00453.2002. [DOI] [PubMed] [Google Scholar]
- 37.Nardone G, Martin G, Rocco A, Rippa E, La Monica G, Caruso F, et al. Molecular expression of gastrokine 1 in normal mucosa and in Helicobacter pylori-related preneoplastic and neoplastic gastric lesions. Cancer Biol Ther. 2008;7(12):1890–5. doi: 10.4161/cbt.7.12.6936. [DOI] [PubMed] [Google Scholar]
- 38.Yoon JH, Song JH, Zhang C, Jin M, Kang YH, Nam SW, et al. Inactivation of the gastrokine 1 gene in gastric adenomas and carcinomas. J Pathol. 2011;223(5):618–25. doi: 10.1002/path.2838. [DOI] [PubMed] [Google Scholar]
- 39.Hnia K, Notarnicola C, de SantaBarbara P, Hugon G, Rivier F, Laoudj-Chenivesse D, et al. Biochemical properties of gastrokine-1 purified from chicken gizzard smooth muscle. PLos One. 2008;3(12):3854. doi: 10.1371/journal.pone.0003854. [DOI] [PMC free article] [PubMed] [Google Scholar]