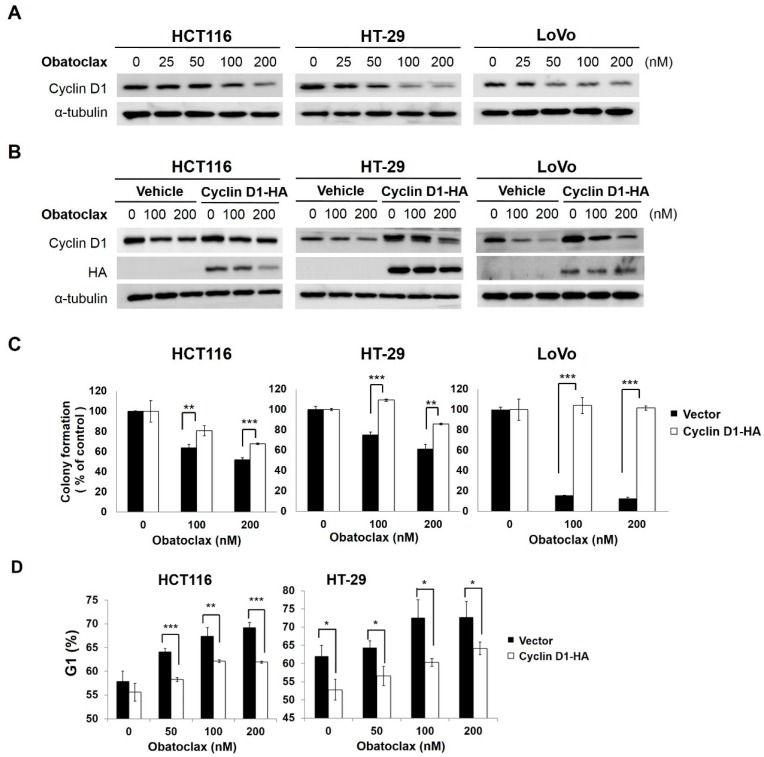
Figure 2.
Obatoclax downregulates cyclin D1 to induce G1-phase arrest and consequent antiproliferation. (A) Obatoclax downregulates cyclin D1. HCT116, HT-29, and LoVo cells were exposed to obatoclax (0, 25, 50, 100, 200 nM) for 24 h, followed by cyclin D1 immunoblotting. α-Tubulin levels served as the control for equal loading; (B) Overexpression of cyclin D1 attenuates obatoclax-elicited reduction of cyclin D1. Stable clones of HCT116, HT-29, and LoVo cells with ectopic expression of hemagglutinin (HA)-tagged cyclin D1 were treated with obatoclax (0, 100, 200 nM) for 24 h. HA immunoblotting was performed to confirm ectopic expression of HA-tagged cyclin D1, and cyclin D1 immunoblotting was used to verify the resistance to the obatoclax-induced decrease in cyclin D1 levels. α-Tubulin levels served as the control for equal loading; (C) Overexpression of cyclin D1 rescues colony formation capability of obatoclax-treated cells. HA-Cyclin D1 stable clones and their corresponding vector controls were treated with obatoclax (0, 100, 200 nM) for 24 h, followed by colony formation assays. Significant restoration of colony numbers was observed in the group with overexpression of HA-Cyclin D1. Data were presented as the percentage relative to the drug-untreated control; (D) Overexpression of cyclin D1 abrogates obatoclax-elicited G1-phase arrest. HA-Cyclin D1 stable clones and their corresponding vector controls were treated with obatoclax (0, 50, 100, 200 nM) for 24 h, followed by cell cycle analysis to determine the levels of cell population at the G1-phase. A marked reduction of cells at the G1 stage was revealed in HA-Cyclin D1 stable clones treated with obatoclax. Data were presented as the percentage relative to the drug-untreated control. *: p < 0.05; **: p < 0.01; ***: p < 0.001.