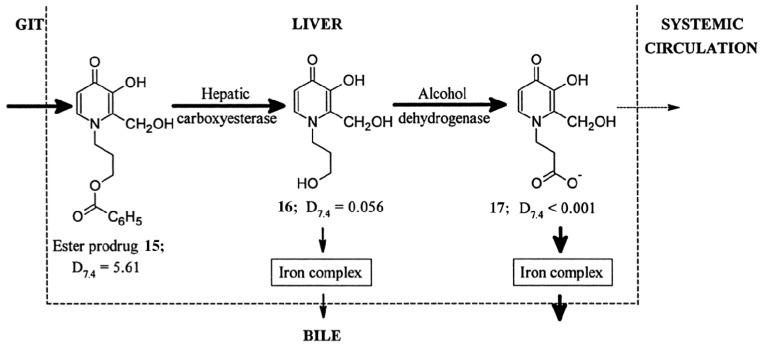
Figure 5.
Schematic representation of the use of hydrophobic esters to enhance both the absorption from the gastrointestinal tract (GIT) and the hepatic extraction of the chelator. Subsequent intracellular hydrolysis occurs yielding a hydrophilic chelator which can undergo further metabolism to the extremely hydrophilic negatively charged 1-carboxyalkyl metabolite in the liver. Reprinted with permission from Liu, Z.D.; et al. Synthesis, physicochemical characterisation, and biological evaluation of 2-(1′-hydroxyalkyl)-3-hydroxypyridin-4-ones: Novel iron chelators with enhanced pFe3+ values. J. Med. Chem. 1999, 42, 4814–4823. Copyright American Chemical Society.