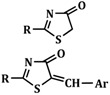
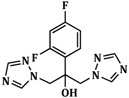
Table 1.
The antifungal activity of the synthesized thiazolin-4-one derivatives (inhibition zone diameters (mm)).
 |
 |
||
|---|---|---|---|
| 2, 5, 8, 10, 11 | Fluconazole | 3a–h, 6a–e, 9a–e | |
| Compound | R | Ar | C. albicans ATCC 10231 |
| 2 | CH2=CH–CH2–NH– | - | 20 ± 0.2 |
| 5 | C6H5–NH– | - | 22 ± 0.5 |
| 8 | α-C10H7–NH– | - | 24 ± 1 |
| 10 | CH3–NH– | - | 22 ± 1 |
| 11 | C6H5– | - | 18 ± 1 |
| 3a | CH2=CH–CH2–NH– |  |
20 ± 0.5 |
| 6a | C6H5–NH– | 20 ± 0.5 | |
| 9a | α-C10H7–NH– | 18 ± 1 | |
| 3b | CH2=CH–CH2–NH– |  |
20 ± 0.5 |
| 6b | C6H5–NH– | 20 ± 1 | |
| 9b | α-C10H7–NH– | 18 ± 0.2 | |
| 3c | CH2=CH–CH2–NH– | 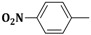 |
18 ± 1 |
| 6c | C6H5–NH– | 20 ± 1 | |
| 9c | α-C10H7–NH– | 20 ± 0.5 | |
| 3d | CH2=CH–CH2–NH– | 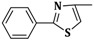 |
20 ± 0.2 |
| 6d | C6H5–NH– | 20 ± 1 | |
| 9d | α-C10H7–NH– | 20 ± 1 | |
| 3e | CH2=CH–CH2–NH– | 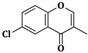 |
20 ± 0.5 |
| 6e | C6H5–NH– | 16 ± 1 | |
| 9e | α-C10H7–NH– | 22 ± 0.5 | |
| 3f | CH2=CH–CH2–NH– | 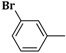 |
22 ± 0.2 |
| 3g | CH2=CH–CH2–NH– | 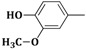 |
22 ± 0.2 |
| 3h | CH2=CH–CH2–NH– | 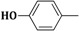 |
20 ± 0.5 |
| Fluconazole | 22 ± 0.5 | ||
The value obtained for each compound represents the mean of three independent measurements ± SD. The values obtained for the most active compounds are marked in bold.
