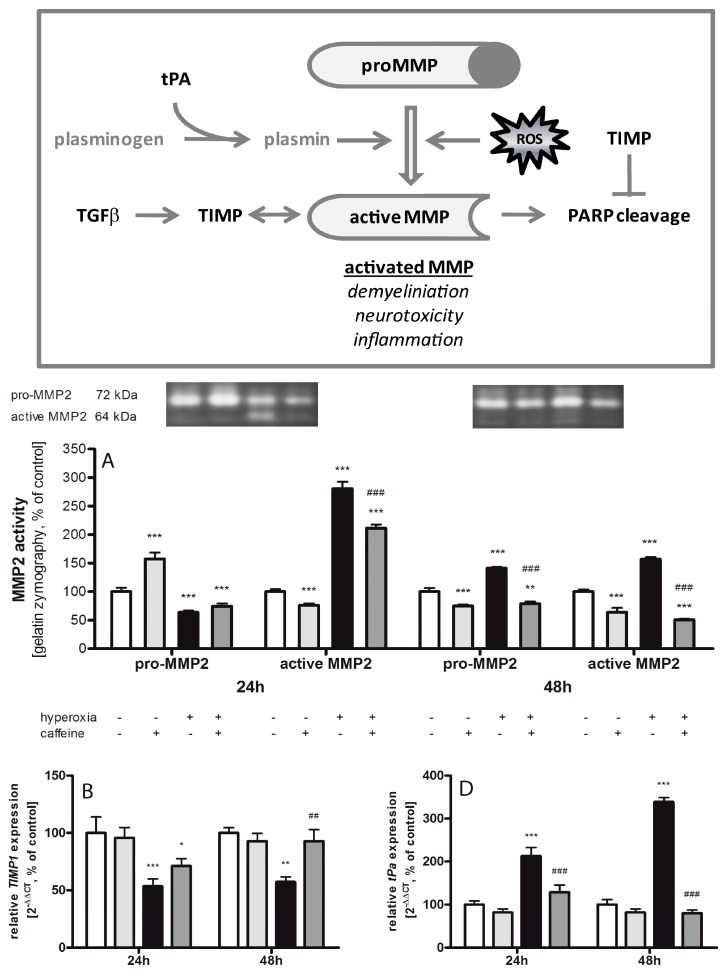
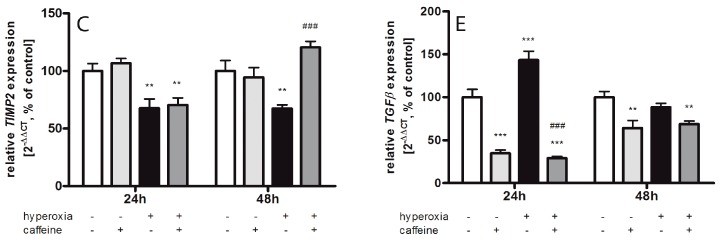
Figure 6.
Hyperoxia-mediated imbalance in the MMP-TIMP system counteracted by caffeine. (Box) The matrix metalloproteinase (MMP) system can be activated via the plasminogen/plasmin system, and ROS. Active MMPs affect a variety of extracellular and immune regulatory proteins and are involved in modulating and degrading processes. Active MMPs can be inhibited by tissue inhibitors of metalloproteinases (TIMPs). TGF-β can enhance the expression of MMPs and TIMPs. MMP2 and MMP9 are able to cleave PARP-1. The MMP inhibitor TIMP2 is able to block PARP-1 degradation. The pro and active MMPs were analyzed with gelatin zymography and relative mRNA expression were measured in brain homogenates of normoxia (white bars), caffeine with normoxia (light grey bars), hyperoxia (black bars), and hyperoxia with caffeine (dark grey bars) by quantitative real-time PCR of: (A) pro/active MMP2; (B,C) TIMP1/2 mRNA; (D) tissue plasminogen activator (tPa) mRNA; and (E) TGF-β mRNA. Data are shown as mean ± SEM, n = 4 (for gelatin zymography)-5 per group per time point. The 100% value is (A) (pro) 7.849 × 106 and 0.949 × 106 intensity/mm2 and (active) 11.18 × 106 and 0.731 × 106 intensity/mm2; (B) 1.043 and 1.004 CT; (C) 1.008 and 1.017 CT; (D) 1.013 and 1.026 CT; and (E) 1.018 and 1.010 CT for 24 and 48 h groups, respectively. Data were analyzed by two-way ANOVA with Bonferroni post hoc test, with * p < 0.05, ** p < 0.01, and *** p < 0.001 versus control (atmospheric air), and ## p < 0.01 and ### p < 0.001 versus hyperoxia (80% oxygen without caffeine).