Abstract
The highly reactive dicarbonyl methylglyoxal (MGO) is mainly formed as byproduct of glycolysis. Therefore, high blood glucose levels determine increased MGO accumulation. Nonetheless, MGO levels are also increased as consequence of the ineffective action of its main detoxification pathway, the glyoxalase system, of which glyoxalase 1 (Glo1) is the rate-limiting enzyme. Indeed, a physiological decrease of Glo1 transcription and activity occurs not only in chronic hyperglycaemia but also with ageing, during which MGO accumulation occurs. MGO and its advanced glycated end products (AGEs) are associated with age-related diseases including diabetes, vascular dysfunction and neurodegeneration. Endothelial dysfunction is the first step in the initiation, progression and clinical outcome of vascular complications, such as retinopathy, nephropathy, impaired wound healing and macroangiopathy. Because of these considerations, studies have been centered on understanding the molecular basis of endothelial dysfunction in diabetes, unveiling a central role of MGO-Glo1 imbalance in the onset of vascular complications. This review focuses on the current understanding of MGO accumulation and Glo1 activity in diabetes, and their contribution on the impairment of endothelial function leading to diabetes-associated vascular damage.
Keywords: methylglyoxal, glyoxalase, vascular function, insulin-resistance
1. Introduction
Methylglyoxal (MGO) is a dicarbonyl aldehyde mainly formed as byproduct of glycolysis [1]. By its nature, MGO efficiently reacts with other molecules in the organism and causes cell and tissue dysfunction [2]. While cells use glucose as major source of energy and store most of their glucose in non-reactive molecules, as glycogen, a spontaneous and unavoidable leakage from the Embden-Meyerhof pathway occurs in mammalian cells in the form of MGO [1]. It has been estimated that about 0.1% of the glucotriose flux generates MGO by the non-enzymatic degradation of glyceraldehyde 3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP) [3]. Thus, glycolysis contributes to the formation of MGO with a rate of about 125 µmol/kg cell mass per day under normoglycaemic conditions [4]. This situation may be further increased under conditions that lead to higher triosephosphate concentrations, like increased glucose metabolism in hyperglycaemia [5], impairment of the pentose pathway decreasing GA3P disposal, or increased anaerobic glycolysis occurring in hypoxia [6]. A minor amount of MGO is generated by acetone oxidation in the catabolism of ketone bodies [7], aminoacetone oxidation in the catabolism of threonine [8], lipid peroxidation and degradation of glycated proteins and monosaccharides [9,10].
At variance, the contribution of exogenous sources is still controversial. Extremely high levels of MGO were found in natural products, such as honey, and especially processed food and beverages like soft drinks, coffee, and dairy products [11,12]. Indeed, MGO levels in foodstuffs is strongly affected by heating [13], prolonged storage and fermentation, when microorganisms release MGO in alcoholic drinks and cheese [11].Unexpectedly, variable levels of MGO can be found even in drinking water that underwent treatments like ozonation and chlorination, which easily result in the formation of MGO from humic substances [14]. It should be further considered that polluted air also represents an exogenous source of MGO. In particular, smoking is a major source of indoor air contamination [15]. However, whether these exogenous sources of MGO are significant for plasma MGO levels has not been clarified yet. Several studies report that free MGO is rapidly degraded during digestion in the intestine and, thus, does not influence MGO level in vivo [16]. But there is also conflicting evidence indicating brain and serum MGO accumulation in rodents fed MGO-supplemented diets and suggesting that at least 10% of MGO-derived AGEs is absorbed and then accumulated in tissues like vessels, heart, liver, kidney and adipose tissue [17,18].
MGO levels in healthy humans have been estimated to be about 50–150 nM in the plasma and 1–4 µM in tissues [19]. When the accumulation of MGO exceeds these levels, dicarbonyl stress occurs as a consequence of the imbalance between the generation/exposure and MGO metabolism [20].
Under physiological conditions, >99% of MGO is detoxified by the glyoxalase system [21], with minor metabolism by aldoketo reductases (AKRs) and aldehyde dehydrogenases (ADHs) which convert MGO to hydroxyacetone and pyruvate, respectively [22]. The glyoxalase system consists of glyoxalase 1 (Glo1), glyoxalase 2 (Glo2) and a catalytic amount of glutathione (GSH). Acting as the rate-limiting enzyme, Glo1 catalyses the primary detoxification step by converting the spontaneously formed MGO-GSH hemithioacetal to the thioester S-d-lactoylglutathione [23]. Its activity is directly proportional to GSH concentration. Thus when cellular GSH concentration declines, as in oxidative stress, Glo1 activity is impaired. Glo2 catalyses the hydrolysis of S-d-lactoylglutathione to d-lactate and GSH [24]. With the exception of certain protozoa [25], both Glo1 and 2 are expressed in most eukaryotes and prokaryotes [26,27], and are localized in the cytoplasmic compartment [28].
The modulation of Glo1 activity is dependent on both regulation of gene expression and post-translational modifications. The Glo1 gene contains regulatory elements including ARE (antioxidant-response element) in exone 1 [29], and its expression is negatively regulated by HIF1α (hypoxia inducible factor 1α) binding to ARE under hypoxic conditions [30]. Indeed, hypoxia is an important physiological driver of dicarbonyl stress as it facilitates MGO formation by increasing metabolic flux through anaerobic glycolysis [6]. Hypoxia decreases Glo1 expression as well [3]. Furthermore, basal and inducible expression of Glo1 is subjected to stress-responsive control by nuclear factor erythroid 2-related factor 2 (Nrf2) through regulatory AREs [29]. Current evidence indicates that NF-κB constitutive activation in chronic hyperglycaemia, and related inflammation, mediates the impairment in Glo1 expression and activity [31]. It has been further demonstrated that NF-κB antagonizes the transcriptional activity of Nrf2 [32], suggesting that in a pro-inflammatory state, typical of diabetes and obesity, NF-κB activation impairs Glo1 expression by inhibiting Nrf2 action. Glo1 expression is also negatively regulated by the activation of the receptor of advanced glycated end products (RAGE) and, again, NF-κB has an important role in this pathway [33].
While genetic polymorphisms of Glo2 are extremely rare, different SNPs (single nucleotide polymorphisms) have been identified in the Glo1 gene and found to be associated with reduced enzyme activity [34], increased prevalence of diabetic neuropathy [35], and increased risk of cardiovascular complications [36]. Moreover, Glo1 is a hotspot for functional copy number variation (CNV) causing 2 to 4-fold increases in Glo1 expression in approximately 2% of the human population [37,38]. However, further studies are needed to understand whether these patients are protected from MGO accumulation and damage.
A physiological decrease of Glo1 activity and expression occurs with ageing, as demonstrated by Morcos et al. in the nematode Caenorhabditis elegans, where an inverse correlation was found between ageing and Glo1 expression [39]. This same effect has been confirmed in rodents [40,41,42]. Glo1 expression is therefore likely linked to healthy ageing [43].
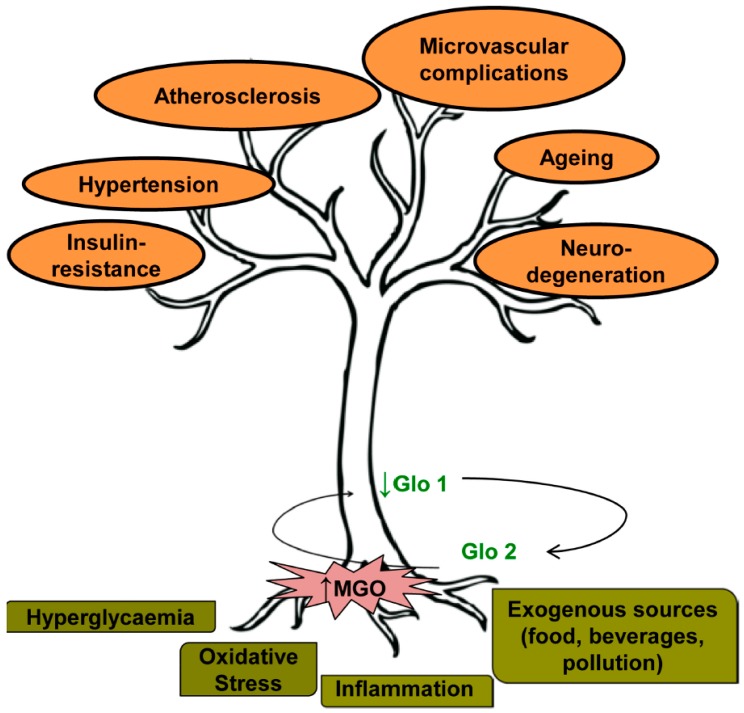
Thus, abnormal cellular accumulation of the dicarbonyl metabolite MGO occurs upon exposure to high glucose concentrations, oxidative stress, inflammation, cell aging and senescence. It is associated with increased MGO-adduct content of proteins susceptible to dicarbonyl modification, collectively defined as dicarbonyl proteome [20], and with DNA instability and mutations [36,43]. An adequate balance between MGO levels and Glo1 activity is necessary to ensure detoxification of MGO from different sources and cell survival (Figure 1).
Figure 1.
Sources of methylglyoxal (MGO) accumulation contributing to vascular dysfunction. Hyperglycaemia, oxidative stress, inflammation and exogenous sources of MGO contribute both to the increase of MGO levels and the decrease of glyoxalase 1 (Glo1) activity. MGO-Glo1 imbalance leads to vascular dysfunction contributing to endothelial insulin-resistance, hypertension, atherosclerosis, microvascular complications, ageing and neuro-degeneration.
2. Effect of MGO Accumulation on Vascular Function
In the last decades, an increasing number of studies claiming a central role for MGO in vascular dysfunction have been published [44]. Endothelial dysfunction is one of the major factors in the development of cardiovascular disease. It has been recently reviewed how different noxious agents induce endothelial dysfunction, promoting an amplified endoplasmic reticulum stress response [45]. Vascular endothelium behaves as an autocrine and paracrine organ regulating vascular homeostasis [46]. This is ensured by the tight control of the vascular tone, cell–cell interaction, permeability and coagulation systems through the production of the extracellular matrix components, and soluble factors in response to various stimuli.
When this balance is impaired, vasoconstriction may occur, along with leukocyte adherence, platelet activation, mitogenesis, pro-oxidation, thrombosis and impaired coagulation, vascular inflammation, and atherosclerosis [47]. Among the bioactive molecules generated by the endothelium, nitric oxide (NO) plays a pivotal role in the maintenance of vascular homeostasis. NO controls vascular tone by maintaining basal and induced vascular smooth muscle relaxation and opposing the action of potent contraction factors, including Angiotensin II (Ang II) and Endothelin-1 (ET-1). In addition, NO inhibits platelet activation and leukocyte adhesion and rolling, as well as cytokine-induced expression of VCAM (vascular cell adhesion molecule) and MCP-1 (monocyte chemotactic protein-1), thus promoting an anti-inflammatory action [47].
MGO accumulation, perpetuated by Glo1 down-regulation, is linked to age-related diseases, such as diabetes, obesity, disorders of the central nervous system and cardiovascular disease, which share endothelial dysfunction as a common pathological denominator [47,48,49,50]. MGO reacts predominantly with arginine residues on proteins, with methylglyoxal hydroimidazolone (MG-H1) being the most prevalent MGO-derived AGE modification found in vivo, leading to structural change, inactivation and degradation of target proteins [51]. Thus, increased levels of MGO impair endothelial function in various way and in different districts in the organism. Several studies have shown an impairment of endothelium-dependent vasorelaxation by MGO, mostly mediated by oxidative stress. Indeed, acetylcholine-induced vasorelaxation is impaired in aortic tissue from rats by both high glucose and MGO. This effect is attenuated by the MGO scavengers aminoguanidine (AG) and N-acetyl-cisteine (NAC) [52], via the inhibition of NADPH oxidase [53], and is prevented by Glo1 over-expression [54]. A protective effect of anti-oxidants such as polyphenols has been reported to prevent MGO-dependent impairment of NO release, improving endothelium-mediated relaxation in mouse corpora cavernosa [55]. A proposed mechanism by which MGO increases reactive oxygen species (ROS), promoting the apoptotic process, involves the reduced transcription of the cytoprotective protein thioredoxin [56]. Moreover, accumulating evidence suggests that physiological angiogenesis is impaired by MGO through RAGE-mediated and autophagy-induced vascular endothelial growth factor receptor 2 (VEGFR2) degradation [57]. Our preliminary data indicate that Glo1 down-regulation in mouse aortic endothelial cells (MAECs) impairs the angiogenic process via a mechanism involving NFκB (unpublished data).
A study performed in Goto-Kakizaki diabetic rats demonstrates that endothelial dysfunction is worsened by MGO treatment, which increases oxidative stress, AGE formation and inflammation with a decline in NO bioavailability [58]. Moreover, MGO accumulation in arterial walls causes vascular contractile dysfunction in spontaneously hypertensive rats [59], and Dhar et al. have demonstrated that MGO treatment activates NFκB through RAGE, thereby increasing renin-angiotensin levels and blood pressure in Sprague-Dawley rats [60]. These findings provide further evidence that MGO is a causative factor in the pathogenesis of atherosclerosis and development of macrovascular diabetic complications. In humans, an association between MGO-derived AGEs and endothelial dysfunction markers has been found in individuals with type 1 diabetes [61,62]. In addition, reduced Glo1 activity in atherosclerotic lesions associates with increased HbA1c levels in non-diabetic patients [63].
As a pivotal mediator of endothelial-dependent release of NO and resulting smooth muscle relaxation, endothelial nitric oxide synthase (eNOS) represents a target of the harmful effect of MGO. Indeed, eNOS protein levels and its active phosphorylated form at the serine 1177 site are decreased in long-term MGO treatment of rat isolated mesenteric artery [53] and in thoracic aortic rings [64]. Also, eNOS uncoupling, found to be associated with O−2 generation and eNOS cofactor biopterin depletion [65], contributes to redox-sensitive leukocyte recruitment and microvascular leakage elicited by MGO [66]. In addition, age-related glycative and oxidative stress, resulting in endothelial dysfunction, is reduced in Glo1 transgenic rats [67].
Besides macrovascular function, MGO and MGO-derived AGEs also play a harmful effect on microvascular function, contributing to the onset of nephropathy and neuropathy. Indeed, regulation of the Glo1 enzyme has been proved to be important in prevention of early renal impairment in experimental diabetes [68], but also independently of hyperglycaemia in apoE−/− mice [69]. This is also confirmed by the evidence that MGO accumulation in Wistar normal rats impairs several renal disease markers progressively observed in diabetic Goto-Kakizaki rats [70]. MGO induces blood–brain barrier damage by reducing the integrity and increasing the permeability of brain endothelial cells [71]. Recently, the generation of an occludin-MGO adduct, which leads to dysfunctional tight junctions and increased brain microvascular endothelial cell (BMEC) permeability, has been identified as a mechanism potentially involved in these abnormalities [72]. BMECs are crucial for brain vascular repair and maintenance. Recent evidence obtained from both in vitro [73,74] and in vivo [75] models of brain ischemia demonstrates that MGO induces BMEC injuries and exaggerates ischemia-reperfusion injury in diabetic rats.
Based on these findings, it became clearer that an effective reduction of MGO accumulation is crucial for preserving vascular function. Several attempts to alleviate dicarbonyl stress have been made in the last few decades, with MGO scavengers such as AG. But trials had to be terminated due to the lack of efficacy, safety concerns or undesired side effects [18]. A promising strategy is the development of Glo1 inducers through the activation and binding of Nrf2 to the Glo1 functional ARE. Up to now, a Glo1 inducer combination of trans-resveratrol and hesperetin (tRES-HESP) has been evaluated in a Phase 1 clinical trial and is now available for evaluation in Phase 2. In highly overweight subjects, tRES-HESP improves arterial dilation and decreases the vascular inflammation marker VCAM-1 [76]. Further efforts in the development of pharmacological intervention to prevent dicarbonyl stress are needed to provide new therapeutic options aimed at preventing vascular dysfunction in diabetes and other age-related diseases.
3. MGO-Induced Insulin-Resistance: A Link to Endothelial Dysfunction
Because of its highly reactive nature, MGO rapidly binds protein residues, with arginine and lysine representing the sites with the higher binding affinity [77]. This is particularly insidious as arginine and lysine are amino acid residues with a high probability of being located in the functional sites of proteins, including kinases involved in insulin signal transduction [3]. We and others have provided evidence about the role of MGO on insulin-resistance in major target tissues for insulin action.
MGO interferes with insulin signaling in L6 skeletal muscle and beta cells. In INS-1E beta cells it decreases insulin secretion through the inhibition of the insulin receptor substrate (IRS)/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway activation and independently from reactive oxygen species production. The inhibitory effect of MGO and the formation of AGE adducts on IRS are reversed by AG [78,79]. In addition, MGO administration in vivo inhibits insulin secretion from isolated beta cells, due to decreased PDX-1 levels, and results in higher levels of MGO-derived AGEs and insulin-resistance in muscle, liver, and adipose tissue [80,81,82]. Such effects are inhibited by the MGO scavenger Alagebrium, indicating the specificity of MGO effects. In support of animal data and studies in animal models, a very recent human study in healthy overweight individuals demonstrates that a diet with low AGE content decreases risk of type 2 diabetes by improving insulin sensitivity [83]. Besides the canonical target tissue of insulin action, MGO induces neuronal cell death in the central nervous system, and the impairment of insulin signaling was found in astrocytes exposed to MGO [84]. In both mice and humans, dietary MGO and AGEs were positively correlated with cognitive deficit, and inversely associated with the survival factor sirtuin-1 (SIRT1) levels and other markers of insulin sensitivity, suggesting MGO dependent SIRT1 down-regulation as a possible link between insulin-resistance and neurodegeneration [17].
Insulin also plays important haemodynamic actions. Insulin receptors were identified on endothelial cells of both large and small blood vessels by Jialal and colleagues in 1985 [85]. The hemodynamic effect of insulin was suggested by pivotal experiments reporting an insulin-mediated increase in blood flow into leg skeletal muscle during a hyperinsulinemic euglycaemic clamp [86]. Indeed, insulin action on endothelium is an integration of increased capillary recruitment and increased total blood flow. Both of these effects are reduced and delayed in insulin-resistance [87].
At the molecular level, insulin binding to its cognate receptor on endothelial cells activates IRS, PI3K and Akt, leading to the activation of eNOS [88]. eNOS catalyses the conversion of arginine to citrulline and NO. Its activity is regulated by phosphorylation at multiple sites; two of the best characterized sites are serine 1177 and threonine 495. Serine 1177 is rapidly phosphorylated by Akt in response to insulin, whereas threonine 495 is constitutively phosphorylated in endothelial cells and is thought to be a negative regulatory site [89,90]. Insulin is also able to cause rapid release of ET-1 via the mitogen-activated protein kinase (MAPK) cascade, which induces the activation of endothelin converting enzyme (ECE) in endothelial cells [50], followed by vasoconstriction and proliferation of vascular smooth muscle cells (VSMCs). Studies enabling the understanding of the causal role of insulin signal transduction in vasodilation were carried out in genetically modified animal models. Mice lacking IRS-1 feature hypertension and impaired endothelium-dependent vasorelaxation [91]. Consistently, a polymorphism at arginine 972 IRS-1 is associated with decreased eNOS expression in endothelial cells and plasma NO levels in human subjects [92]. Moreover, eNOS knock-out mice are hypertensive and have a 40% lower insulin-stimulated glucose uptake than control mice [93], indicating that insulin-mediated vasodilation and capillary recruitment are required for insulin delivery and action at target tissues.
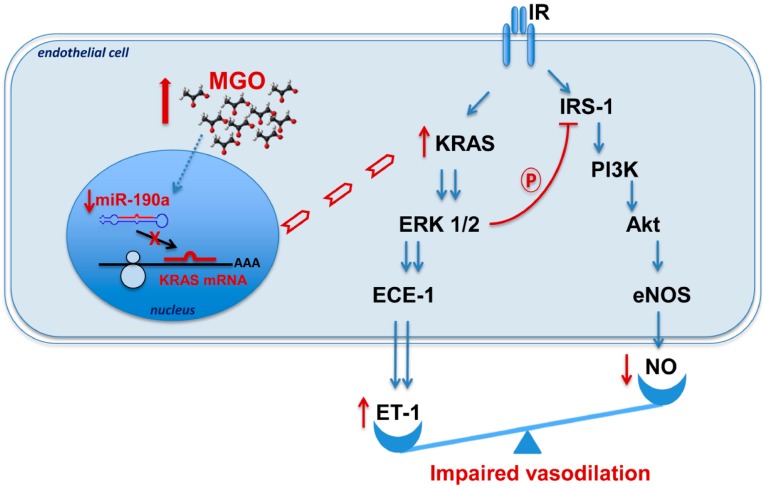
Besides the above mentioned harmful effect of MGO on insulin-sensitivity of insulin target tissues, we have recently identified MGO as an endothelial insulin-resistance-inducing molecule (Figure 2) [94]. Increased MGO levels are responsible for the impairment of insulin action in mouse aortic endothelial cells (MAECs) exposed to exogenous MGO, or treated with the Glo1 inhibitor S-p-bromobenzylglutathione cyclopentyl diester (SpBrBzGSHCp2), and in vivo in healthy mice chronically treated with MGO. MGO exposure renders insulin unable to induce IRS-1 tyrosine phosphorylation, its association to p85, Akt activation and the subsequent eNOS phosphorylation on serine 1177, while the inhibitory phosphorylation of eNOS on threonine 497 is prevented by MGO accumulation. eNOS inactivation results in loss of the insulin-dependent increase in NO production. Conversely, ERK 1/2 activation is enhanced by MGO in both MAECs and aortic tissue from MGO-treated mice, accompanied by increased phosphorylation of IRS-1 on its inhibitory site serine 616. The rescue of IRS-1/Akt/eNOS pathway activation following the chemical inhibition of ERK 1/2 indicates that ERK 1/2 mediates, at least in part, the detrimental effect exerted by MGO on IRS-1/Akt pathway activation by phosphorylating IRS-1 at serine 616. Furthermore, ERK 1/2 hyperactivation does not result in increased MAEC proliferation, but rather in the increase of ET-1 production both in the absence and presence of insulin, suggesting that MGO alters endothelial insulin signaling and causes an imbalance between NO and ET-1 production, which facilitates vasoconstriction. Studies by Raoch et al. showed that NO regulates endothelin converting enzyme 1 ECE-1 expression [95]. It is therefore possible that the reduced NO levels also contribute to the activation of ECE-1 and the subsequent release of ET-1.
Figure 2.
MGO-mediated endothelial insulin-resistance. MGO accumulation causes the reduction of miR-190a. Protein levels of miR-190a target KRAS increased, resulting in the hyperactivation of ERK 1/2. The latter phosphorylates IRS-1 (insulin receptor substrate 1) on serine 616 inhibiting its activation and the downstream pathway PI3K/Akt/eNOS. These effects result in the impairment of insulin stimulated NO production by endothelial cells, together with increased ET-1 release. Red ↑: increased molecule levels; red ↓: decreased molecule levels; blue ↓: protein activation; blue ↓↓: protein hyperactivation. KRAS (Kirsten rat sarcoma viral oncogene homolog).
Emerging evidence suggests that MGO may alter gene expression through miRNA regulation [96,97], besides changes in DNA methylation [98] and histone modifications [99,100]. To answer the question of how MGO increases ERK 1/2 activation in MAECs, we have carried on studies which led to the recognition of miR-190a as an important modulator. The expression of this miRNA is down-regulated in both MAECs treated with MGO and aortic tissue from mice where Glo1 expression has been knocked down (Glo1-KD mice) [101]. The modulation of miR-190a inversely correlated with Kirsten rat sarcoma viral oncogene homolog (KRAS) levels and ERK 1/2 activation. Furthermore, pretreatment of MAECs with the inhibitor of class I and II histone deacetylase (HDAC), trichostatin A, was able to abolish the MGO effect on miR-190a expression, suggesting HDAC involvement. Consistent with previous findings proving the role of MGO in insulin-sensitivity and vascular function [53,55,78], the rescue of miR-190a levels we observed in MAECs upon the co-treatment with MGO and AG, revealed that MGO-modified proteins are critical in determining MGO-induced down-regulation of miR-190a.
Despite demonstration that MGO promotes insulin-resistance and hypertension by increasing oxidative stress [81], other investigators reported MGO-mediated insulin-resistance in skeletal muscle cells in the absence of ROS increases [79]. Whether oxidative stress may also play a part in MGO-mediated endothelial insulin-resistance remains to be further addressed.
4. Mouse Models of MGO Accumulation
To study the impact of MGO on vascular function, animal models that allow observation of the systemic implications of an imbalanced accumulation/detoxification ratio of MGO have been investigated. Important insights in this field have been generated by studies carried out in rodents that received MGO in drinking water [81], subcutaneous infusion with osmotic minipumps [80] or by chronic intraperitoneal infusion [94,102]. However, exogenous sources of MGO are only partially absorbed and not completely accumulated in tissues as free MGO, thus limiting the interpretation of these studies. Indeed, MGO rapidly reacts generating in situ adducts that, depending on the nature and half-life of the specific protein, can move in different ways to other districts in the organism or remain confined to the site of administration [22]. Experimental studies have often used MGO levels 10–20-fold higher than physiological concentrations. These conditions are similar to or exceed the upper limit of severe dicarbonyl stress in poorly controlled diabetes [103]. Moreover, MGO is physiologically formed intracellularly, as also indicated by the cellular MGO concentration exceeding that in plasma of human subjects by 100-fold [104]. Measuring tissue and plasma levels in experimental models is therefore necessary to ensure the patho-physiological relevance of the animal model.
A possibility to bypass these limitations is to modulate Glo1 activity and/or expression. Indeed, reduced activity of Glo1, inducible by treatment with the chemical Glo1 inhibitor SpBrBzGSHCp2 or by silencing Glo1 expression [105,106], mimics the physiological condition of endogenous accumulation of MGO as a consequence of MGO/Glo1 imbalance, occurring in both diabetes and ageing [107]. Conversely, Glo1 over-expression reduces hyperglycaemia-induced carbonyl stress [108], and has a protective effect on vascular dysfunction [68]. Chemical inhibition of Glo1 in apoE−/− mice is able to increase endothelial inflammation and atherogenesis in the absence of hyperglycaemia, to a similar extent as in hyperglycaemic mice with diabetes [109]. Other studies using Glo1 genetically modified mice demonstrate its implication in microvascular complications. As reported by Giacco F. and colleagues, modulation of Glo1 in mice influences the risk of nephropathy development [106]. Indeed, in non-diabetic mice, knockdown of Glo1 increases both MGO modification of glomerular proteins and oxidative stress to diabetic levels, and causes alterations in kidney morphology which are indistinguishable from those caused by diabetes. Conversely, in streptozotocin (STZ)-induced diabetic mice, Glo1 over-expression prevents diabetes-induced increases in MGO modification of glomerular proteins, increased oxidative stress, and the development of diabetic kidney pathology, despite unchanged levels of diabetic hyperglycemia [106]. Recently, beneficial effects of Glo1 over-expression were also found in tubular cell survival, resulting in nephroprotective effects in renal ischemia-perfusion injury [110]. Another study of Bierhaus A. et al., demonstrated that both chemical Glo1 inhibition in wild-type mice and non-diabetic Glo1 knockdown mice develop signs of peripheral neuropathy, including thermal and mechanical hyperalgesia [105]. Strain dependent differences in Glo1 copy numbers also confirm that a lower expression of Glo1 promotes the development of diabetic neuropathy symptoms [111]. Very recent evidence obtained in Glo1 over-expressing mice has shown that MGO increases inflammation in diabetes, leading to endothelial cell loss and, thus, contributing to the development of diabetic cardiomyopathy [112]. Moreover, systemic Glo1 over-expression in rats prevents age-related impairment of endothelium-dependent vasorelaxation through modulation of eNOS phosphorylation, proving that blunting glycative stress prevents the long-term impact of endothelial dysfunction on vascular ageing [67]. In line with these findings, our preliminary data obtained in Glo1 knockdown mice indicated that Glo1 down-regulation is sufficient to increase blood pressure with ageing, likely as a consequence of impaired endothelium-dependent vasorelaxation. By contrast, previous studies in the same model did not reveal any differences in blood pressure compared to wild-type mice [106]. In this study, however, blood pressure was recorded in young mice where, even in presence of early signs of kidney damage, hypertension seemed to be not clinically evident yet.
5. Conclusions
It is undeniable that MGO accumulation has harmful effects on vascular function, by inducing insulin-resistance, hypertension, atherosclerosis, neurodegenerative disease and diabetic microvascular complications. A large body of literature supports the concept that maintaining the MGO/Glo1 balance is crucial to guarantee the containment of MGO levels under the toxic threshold. To this purpose, it is necessary to adopt a multi-task strategy. It is indeed not sufficient to minimize MGO intake. Also, improving glucose control to treat vascular dysfunction is likely hampered by metabolic memory. In this context, a promising contribution might be provided by modulating Glo1 activity. At the moment, it is necessary to use optimal experimental models to further uncover the pathways contributing to MGO accumulation and its effects, as well as its real impact on physiological systems, fostering the development of novel and effective pharmacological interventions to prevent vascular dysfunction.
Acknowledgments
This work was supported by the European Foundation for the Studies of Diabetes (EFSD)/Novo Nordisk (2015-2017), by the Ministero dell’Università e della Ricerca Scientifica (grants PRIN).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Phillips S.A., Thornalley P.J. The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur. J. Biochem. 1993;212:101–105. doi: 10.1111/j.1432-1033.1993.tb17638.x. [DOI] [PubMed] [Google Scholar]
- 2.Rabbani N., Xue M., Thornalley P.J. Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj. J. 2016;33:513–525. doi: 10.1007/s10719-016-9705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabbani N., Xue M., Thornalley P.J. Methylglyoxal-induced dicarbonyl stress in aging and disease: First steps towards glyoxalase 1-based treatments. Clin. Sci. 2016;130:1677–16796. doi: 10.1042/CS20160025. [DOI] [PubMed] [Google Scholar]
- 4.Thornalley P.J. Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem. J. 1988;254:751–755. doi: 10.1042/bj2540751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babaei-Jadidi R., Karachalias N., Ahmed N., Battah S., Thornalley P.J. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 2003;52:2110–2120. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- 6.Seagroves T.N., Ryan H.E., Lu H., Wouters B.G., Knapp M., Thibault P., Laderoute K., Johnson R.S. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol. Cell. Biol. 2001;21:3436–3444. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beisswenger B.G., Delucia E.M., Lapoint N., Sanford R.J., Beisswenger P.J. Ketosis leads to increased methylglyoxal production on the Atkins diet. Ann. N. Y. Acad. Sci. 2005;1043:201–210. doi: 10.1196/annals.1333.025. [DOI] [PubMed] [Google Scholar]
- 8.Lyles G.A., Chalmers J. The metabolism of aminoacetone to methylglyoxal by semicarbazide-sensitive amine oxidase in human umbilical artery. Biochem. Pharmacol. 1992;43:1409–1414. doi: 10.1016/0006-2952(92)90196-P. [DOI] [PubMed] [Google Scholar]
- 9.Kalapos M.P. Methylglyoxal in living organisms: Chemistry, biochemistry, toxicology and biological implications. Toxicol. Lett. 1999;110:145–175. doi: 10.1016/S0378-4274(99)00160-5. [DOI] [PubMed] [Google Scholar]
- 10.Thornalley P.J., Langborg A., Minhas H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999;344(Pt 1):109–116. doi: 10.1042/bj3440109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemet I., Varga-Defterdarovic L., Turk Z. Methylglyoxal in food and living organisms. Mol. Nutr. Food. Res. 2006;50:1105–1117. doi: 10.1002/mnfr.200600065. [DOI] [PubMed] [Google Scholar]
- 12.Degen J., Hellwig M., Henle T. 1,2-dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012;60:7071–7079. doi: 10.1021/jf301306g. [DOI] [PubMed] [Google Scholar]
- 13.Martins S.I., van Boekel M.A. Kinetic modelling of Amadori N-(1-deoxy-d-fructos-1-yl)-glycine degradation pathways. Part II—kinetic analysis. Carbohydr. Res. 2003;338:1665–1678. doi: 10.1016/S0008-6215(03)00174-5. [DOI] [PubMed] [Google Scholar]
- 14.Do Rosario P.M., Cordeiro C.A., Freire A.P., Nogueira J.M. Analysis of methylglyoxal in water and biological matrices by capillary zone electrophoresis with diode array detection. Electrophoresis. 2005;26:1760–1767. doi: 10.1002/elps.200410216. [DOI] [PubMed] [Google Scholar]
- 15.Fujioka K., Shibamoto T. Determination of toxic carbonyl compounds in cigarette smoke. Environ. Toxicol. 2006;21:47–54. doi: 10.1002/tox.20153. [DOI] [PubMed] [Google Scholar]
- 16.Degen J., Vogel M., Richter D., Hellwig M., Henle T. Metabolic transit of dietary methylglyoxal. J. Agric. Food Chem. 2013;61:10253–10260. doi: 10.1021/jf304946p. [DOI] [PubMed] [Google Scholar]
- 17.Cai W., Uribarri J., Zhu L., Chen X., Swamy S., Zhao Z., Grosjean F., Simonaro C., Kuchel G.A., Schnaider-Beeri M., et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc. Natl. Acad. Sci. USA. 2014;111:4940–4945. doi: 10.1073/pnas.1316013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maessen D.E., Stehouwer C.D., Schalkwijk C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015;128:839–861. doi: 10.1042/CS20140683. [DOI] [PubMed] [Google Scholar]
- 19.Rabbani N., Thornalley P.J. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat. Protoc. 2014;9:1969–1979. doi: 10.1038/nprot.2014.129. [DOI] [PubMed] [Google Scholar]
- 20.Rabbani N., Thornalley P.J. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun. 2015;458:221–226. doi: 10.1016/j.bbrc.2015.01.140. [DOI] [PubMed] [Google Scholar]
- 21.Rabbani N., Thornalley P.J. Dicarbonyl proteome and genome damage in metabolic and vascular disease. Biochem. Soc. Trans. 2014;42:425–432. doi: 10.1042/BST20140018. [DOI] [PubMed] [Google Scholar]
- 22.Baba S.P., Barski O.A., Ahmed Y., O’Toole T.E., Conklin D.J., Bhatnagar A., Srivastava S. Reductive metabolism of AGE precursors: A metabolic route for preventing AGE accumulation in cardiovascular tissue. Diabetes. 2009;58:2486–2497. doi: 10.2337/db09-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sousa Silva M., Gomes R.A., Ferreira A.E., Ponces Freire A., Cordeiro C. The glyoxalase pathway: The first hundred years and beyond. Biochem. J. 2013;453:1–15. doi: 10.1042/BJ20121743. [DOI] [PubMed] [Google Scholar]
- 24.Thornalley P.J. The glyoxalase system in health and disease. Mol. Aspects. Med. 1993;14:287–371. doi: 10.1016/0098-2997(93)90002-U. [DOI] [PubMed] [Google Scholar]
- 25.Silva M.S., Ferreira A.E., Gomes R., Tomás A.M., Freire A.P., Cordeiro C. The glyoxalase pathway in protozoan parasites. Int. J. Med. Microbiol. 2012;302:225–229. doi: 10.1016/j.ijmm.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Aronsson A.C., Marmstal E., Mannervik B. Glyoxalase I, a zinc metalloenzyme of mammals and yeast. Biochem. Biophys. Res. Commun. 1978;81:1235–1240. doi: 10.1016/0006-291X(78)91268-8. [DOI] [PubMed] [Google Scholar]
- 27.He M.M., Clugston S.L., Honek J.F., Matthews B.W. Determination of the structure of Escherichia coli glyoxalase I suggests a structural basis for differential metal activation. Biochemistry. 2000;39:8719–8727. doi: 10.1021/bi000856g. [DOI] [PubMed] [Google Scholar]
- 28.Rabbani N., Thornalley P.J. Glyoxalase, Centennial conference: Introduction, history of research on the glyoxalase system and future prospects. Biochem. Soc. Trans. 2014;42:413–418. doi: 10.1042/BST20140014. [DOI] [PubMed] [Google Scholar]
- 29.Xue M., Rabbani N., Momiji H., Imbasi P., Anwar M.M., Kitteringham N., Park B.K., Souma T., Moriguchi T., Yamamoto M., et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012;443:213–222. doi: 10.1042/BJ20111648. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H., Li H., Xi H.S., Li S. HIF1α is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119:2595–2607. doi: 10.1182/blood-2011-10-387381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bierhaus A., Schiekofer S., Schwaninger M., Andrassy M., Humpert P.M., Chen J., Hong M., Luther T., Henle T., Klöting I., et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 32.Liu G.H., Qu J., Shen X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Rabbani N., Xue M., Thornalley P.J. Activity, regulation, copy number and function in the glyoxalase system. Biochem. Soc. Trans. 2014;42:419–424. doi: 10.1042/BST20140008. [DOI] [PubMed] [Google Scholar]
- 34.Peculis R., Konrade I., Skapare E., Fridmanis D., Nikitina-Zake L., Lejnieks A., Pirags V., Dambrova M., Klovins J. Identification of glyoxalase 1 polymorphisms associated with enzyme activity. Gene. 2013;515:140–143. doi: 10.1016/j.gene.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Groener J.B., Reismann P., Fleming T., Kalscheuer H., Lehnhoff D., Hamann A., Roser P., Bierhaus A., Nawroth P.P., Rudofsky G. C332C genotype of glyoxalase 1 and its association with late diabetic complications. Exp. Clin. Endocrinol. Diabetes. 2013;121:436–439. doi: 10.1055/s-0033-1345124. [DOI] [PubMed] [Google Scholar]
- 36.Rabbani N., Thornalley P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell Dev. Biol. 2011;22:309–317. doi: 10.1016/j.semcdb.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Cahan P., Li Y., Izumi M., Graubert T.A. The impact of copy number variation on local gene expression in mouse hematopoietic stem and progenitor cells. Nat. Genet. 2009;41:430–437. doi: 10.1038/ng.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redon R., Ishikawa S., Fitch K.R., Feuk L., Perry G.H., Andrews T.D., Fiegler H., Shapero M.H., Carson A.R., Chen W., et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morcos M., Du X., Pfisterer F., Hutter H., Sayed A.A., Thornalley P., Ahmed N., Baynes J., Thorpe S., Kukudov G., et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell. 2008;7:260–269. doi: 10.1111/j.1474-9726.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- 40.Fleming T.H., Theilen T.M., Masania J., Wunderle M., Karimi J., Vittas S., Bernauer R., Bierhaus A., Rabbani N., Thornalley P.J., et al. Aging-dependent reduction in glyoxalase 1 delays wound healing. Gerontology. 2013;59:427–437. doi: 10.1159/000351628. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda Y., Inagi R., Miyata T., Nagai R., Arai M., Miyashita M., Itokawa M., Fujita T., Nangaku M. Glyoxalase I retards renal senescence. Am. J. Pathol. 2011;179:2810–2821. doi: 10.1016/j.ajpath.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu Q., Wang B., Zhang X.F., Ma Y.P., Liu J.D., Wang X.Z. Contribution of receptor for advanced glycation end products to vasculature-protecting effects of exercise training in aged rats. Eur. J. Pharmacol. 2014;741:186–194. doi: 10.1016/j.ejphar.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Xue M., Rabbani N., Thornalley P.J. Glyoxalase in ageing. Semin. Cell Dev. Biol. 2011;22:293–301. doi: 10.1016/j.semcdb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Shamsaldeen Y.A., Mackenzie L.S., Lione L.A., Benham C.D. Methylglyoxal, A Metabolite Increased in Diabetes is Associated with Insulin Resistance, Vascular Dysfunction and Neuropathies. Curr. Drug Metab. 2016;17:359–367. doi: 10.2174/1389200217666151222155216. [DOI] [PubMed] [Google Scholar]
- 45.Cimellaro A., Perticone M., Fiorentino T.V., Sciacqua A., Hribal M.L. Role of endoplasmic reticulum stress in endothelial dysfunction. Nutr. Metab. Cardiovasc. Dis. 2016;26:863–871. doi: 10.1016/j.numecd.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Szmitko P.E., Wang C.H., Weisel R.D., de Almeida J.R., Anderson T.J., Verma S. New markers of inflammation and endothelial cell activation: Part I. Circulation. 2003;108:1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 47.Verma S., Anderson T.J. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 48.Dhananjayan R., Koundinya K.S., Malati T., Kutala V.K. Endothelial Dysfunction in Type 2 Diabetes Mellitus. Indian J. Clin. Biochem. 2016;31:372–379. doi: 10.1007/s12291-015-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimassi S., Chahed K., Boumiza S., Canault M., Tabka Z., Laurant P., Riva C. Role of eNOS- and NOX-containing microparticles in endothelial dysfunction in patients with obesity. Obesity. 2016;24:1305–1312. doi: 10.1002/oby.21508. [DOI] [PubMed] [Google Scholar]
- 50.Eringa E.C., Stehouwer C.D., van Nieuw Amerongen G.P., Ouwehand L., Westerhof N., Sipkema P. Vasoconstrictor effects of insulin in skeletal muscle arterioles are mediated by ERK1/2 activation in endothelium. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2043–H2048. doi: 10.1152/ajpheart.00067.2004. [DOI] [PubMed] [Google Scholar]
- 51.Schalkwijk C.G. Vascular AGE-ing by methylglyoxal: The past, the present and the future. Diabetologia. 2015;58:1715–1719. doi: 10.1007/s00125-015-3597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhar A., Dhar I., Desai K.M., Wu L. Methylglyoxal scavengers attenuate endothelial dysfunction induced by methylglyoxal and high concentrations of glucose. Br. J. Pharmacol. 2010;161:1843–1856. doi: 10.1111/j.1476-5381.2010.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukohda M., Morita T., Okada M., Hara Y., Yamawaki H. Long-term methylglyoxal treatment causes endothelial dysfunction of rat isolated mesenteric artery. J. Vet. Med. Sci. 2013;75:151–157. doi: 10.1292/jvms.12-0345. [DOI] [PubMed] [Google Scholar]
- 54.Brouwers O., Niessen P.M., Haenen G., Miyata T., Brownlee M., Stehouwer C.D., de Mey J.G., Schalkwijk C.G. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010;53:989–1000. doi: 10.1007/s00125-010-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boydens C., Pauwels B., van den Daele L., van de Voorde J. Protective effect of resveratrol and quercetin on in vitro-induced diabetic mouse corpus cavernosum. Cardiovasc. Diabetol. 2016;15:46. doi: 10.1186/s12933-016-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oba T., Tatsunami R., Sato K., Takahashi K., Hao Z., Tampo Y. Methylglyoxal has deleterious effects on thioredoxin in human aortic endothelial cells. Environ. Toxicol. Pharmacol. 2012;34:117–126. doi: 10.1016/j.etap.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Liu H., Yu S., Zhang H., Xu J. Angiogenesis impairment in diabetes: Role of methylglyoxal-induced receptor for advanced glycation endproducts, autophagy and vascular endothelial growth factor receptor 2. PLoS ONE. 2012;7:e46720. doi: 10.1371/journal.pone.0046720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sena C.M., Matafome P., Crisostomo J., Rodrigues L., Fernandes R., Pereira P., Seica R.M. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol. Res. 2012;65:497–506. doi: 10.1016/j.phrs.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Mukohda M., Okada M., Hara Y., Yamawaki H. Methylglyoxal accumulation in arterial walls causes vascular contractile dysfunction in spontaneously hypertensive rats. J. Pharmacol. Sci. 2012;120:26–35. doi: 10.1254/jphs.12088FP. [DOI] [PubMed] [Google Scholar]
- 60.Dhar I., Dhar A., Wu L., Desai K.M. Methylglyoxal, a reactive glucose metabolite, increases renin angiotensin aldosterone and blood pressure in male Sprague-Dawley rats. Am. J. Hypertens. 2014;27:308–316. doi: 10.1093/ajh/hpt281. [DOI] [PubMed] [Google Scholar]
- 61.Van Eupen M.G., Schram M.T., Colhoun H.M., Hanssen N.M., Niessen H.W., Tarnow L., Parving H.H., Rossing P., Stehouwer C.D.A., Schalkwijk C.G. The methylglyoxal-derived AGE tetrahydropyrimidine is increased in plasma of individuals with type 1 diabetes mellitus and in atherosclerotic lesions and is associated with sVCAM-1. Diabetologia. 2013;56:1845–1855. doi: 10.1007/s00125-013-2919-8. [DOI] [PubMed] [Google Scholar]
- 62.Heier M., Margeirsdottir H.D., Torjesen P.A., Seljeflot I., Stensaeth K.H., Gaarder M., Brunborg C., Hanssen K.F., Dahl-Jørgensen K. The advanced glycation end product methylglyoxal-derived hydroimidazolone-1 and early signs of atherosclerosis in childhood diabetes. Diab. Vasc. Dis. Res. 2015;12:139–145. doi: 10.1177/1479164114560910. [DOI] [PubMed] [Google Scholar]
- 63.Peters A.S., Lercher M., Fleming T.H., Nawroth P.P., Bischoff M.S., Dihlmann S., Böckler D., Hakimi M. Reduced glyoxalase 1 activity in carotid artery plaques of nondiabetic patients with increased hemoglobin A1c level. J. Vasc. Surg. 2016;64:990–994. doi: 10.1016/j.jvs.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 64.Turkseven S., Ertuna E., Yetik-Anacak G., Yasa M. Methylglyoxal causes endothelial dysfunction: The role of endothelial nitric oxide synthase and AMP-activated protein kinase α. J. Basic. Clin. Physiol. Pharmacol. 2014;25:109–115. doi: 10.1515/jbcpp-2013-0095. [DOI] [PubMed] [Google Scholar]
- 65.Ravassa S., Barba J., Coma-Canella I., Huerta A., Lopez B., Gonzalez A., Díez J. The activity of circulating dipeptidyl peptidase-4 is associated with subclinical left ventricular dysfunction in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2013;12:143. doi: 10.1186/1475-2840-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su Y., Qadri S.M., Hossain M., Wu L., Liu L. Uncoupling of eNOS contributes to redox-sensitive leukocyte recruitment and microvascular leakage elicited by methylglyoxal. Biochem. Pharmacol. 2013;86:1762–1774. doi: 10.1016/j.bcp.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 67.Jo-Watanabe A., Ohse T., Nishimatsu H., Takahashi M., Ikeda Y., Wada T., Shirakawa J., Nagai R., Miyata T., Nagano T., et al. Glyoxalase I reduces glycative and oxidative stress and prevents age-related endothelial dysfunction through modulation of endothelial nitric oxide synthase phosphorylation. Aging Cell. 2014;13:519–528. doi: 10.1111/acel.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brouwers O., Niessen P.M., Miyata T., Ostergaard J.A., Flyvbjerg A., Peutz-Kootstra C.J., Sieber J., Mundel P.H., Brownlee M., Janssen B.J., et al. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia. 2014;57:224–235. doi: 10.1007/s00125-013-3088-5. [DOI] [PubMed] [Google Scholar]
- 69.Geoffrion M., Du X., Irshad Z., Van der hyden B.C., Courville K., Sui G., D'Agati V.D., Ott-Braschi S., Rabbani N., Thornalley P.J., et al. Differential effects of glyoxalase 1 overexpression on diabetic atherosclerosis and renal dysfunction in streptozotocin-treated, apolipoprotein E-deficient mice. Physiol. Rep. 2014;2:e12043. doi: 10.14814/phy2.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodrigues L., Matafome P., Crisostomo J., Santos-Silva D., Sena C., Pereira P., Seiça R. Advanced glycation end products and diabetic nephropathy: A comparative study using diabetic and normal rats with methylglyoxal-induced glycation. J. Physiol. Biochem. 2014;70:173–184. doi: 10.1007/s13105-013-0291-2. [DOI] [PubMed] [Google Scholar]
- 71.Toth A.E., Walter F.R., Bocsik A., Santha P., Veszelka S., Nagy L., Puskás L.G., Couraud P.O., Takata F., Dohgu S., et al. Edaravone protects against methylglyoxal-induced barrier damage in human brain endothelial cells. PLoS ONE. 2014;9:e100152. doi: 10.1371/journal.pone.0100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W., Maloney R.E., Aw T.Y. High glucose, glucose fluctuation and carbonyl stress enhance brain microvascular endothelial barrier dysfunction: Implications for diabetic cerebral microvasculature. Redox. Biol. 2015;5:80–90. doi: 10.1016/j.redox.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li W., Chen Z., Yan M., He P., Chen Z., Dai H. The protective role of isorhamnetin on human brain microvascular endothelial cells from cytotoxicity induced by methylglyoxal and oxygen-glucose deprivation. J. Neurochem. 2016;136:651–659. doi: 10.1111/jnc.13436. [DOI] [PubMed] [Google Scholar]
- 74.Fang L., Li X., Zhong Y., Yu J., Yu L., Dai H., Yan M. Autophagy protects human brain microvascular endothelial cells against methylglyoxal-induced injuries, reproducible in a cerebral ischemic model in diabetic rats. J. Neurochem. 2015;135:431–440. doi: 10.1111/jnc.13277. [DOI] [PubMed] [Google Scholar]
- 75.Alomar F., Singh J., Jang H.S., Rozanzki G.J., Shao C.H., Padanilam B.J., Mayhan W.G., Bidasee K.R. Smooth muscle-generated methylglyoxal impairs endothelial cell-mediated vasodilatation of cerebral microvessels in type 1 diabetic rats. Br. J. Pharmacol. 2016 doi: 10.1111/bph.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xue M., Weickert M.O., Qureshi S., Kandala N.B., Anwar A., Waldron M., Shafie A., Messenger D., Fowler M., Jenkins G., et al. Improved Glycemic Control and Vascular Function in Overweight and Obese Subjects by Glyoxalase 1 Inducer Formulation. Diabetes. 2016;65:2282–2294. doi: 10.2337/db16-0153. [DOI] [PubMed] [Google Scholar]
- 77.Rabbani N., Thornalley P.J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino. Acids. 2012;42:1133–1142. doi: 10.1007/s00726-010-0783-0. [DOI] [PubMed] [Google Scholar]
- 78.Fiory F., Lombardi A., Miele C., Giudicelli J., Beguinot F., van Obberghen E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia. 2011;54:2941–2952. doi: 10.1007/s00125-011-2280-8. [DOI] [PubMed] [Google Scholar]
- 79.Riboulet-Chavey A., Pierron A., Durand I., Murdaca J., Giudicelli J., Van Obberghen E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes. 2006;55:1289–1299. doi: 10.2337/db05-0857. [DOI] [PubMed] [Google Scholar]
- 80.Dhar A., Dhar I., Jiang B., Desai K.M., Wu L. Chronic methylglyoxal infusion by minipump causes pancreatic beta-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes. 2011;60:899–908. doi: 10.2337/db10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo Q., Mori T., Jiang Y., Hu C., Osaki Y., Yoneki Y., Sun Y., Hosoya T., Kawamata A., Ogawa S., et al. Methylglyoxal contributes to the development of insulin resistance and salt sensitivity in Sprague-Dawley rats. J. Hypertens. 2009;27:1664–1671. doi: 10.1097/HJH.0b013e32832c419a. [DOI] [PubMed] [Google Scholar]
- 82.Cai W., Ramdas M., Zhu L., Chen X., Striker G.E., Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc. Natl. Acad. Sci. USA. 2012;109:15888–15893. doi: 10.1073/pnas.1205847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Courten B., de Courten M.P., Soldatos G., Dougherty S.L., Straznicky N., Schlaich M., Sourris K.C., Chand V., Scheijen J.L., Kingwell B.A., et al. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: A double-blind, randomized, crossover trial. Am. J. Clin. Nutr. 2016;103:1426–1433. doi: 10.3945/ajcn.115.125427. [DOI] [PubMed] [Google Scholar]
- 84.Chu J.M., Lee D.K., Wong D.P., Wong R.N., Yung K.K., Cheng C.H., Yue K.K. Ginsenosides attenuate methylglyoxal-induced impairment of insulin signaling and subsequent apoptosis in primary astrocytes. Neuropharmacology. 2014;85:215–223. doi: 10.1016/j.neuropharm.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 85.Jialal I., Crettaz M., Hachiya H.L., Kahn C.R., Moses A.C., Buzney S.M., King G.L. Characterization of the receptors for insulin and the insulin-like growth factors on micro and macrovascular tissues. Endocrinology. 1985;117:1222–1229. doi: 10.1210/endo-117-3-1222. [DOI] [PubMed] [Google Scholar]
- 86.Baron A.D. Hemodynamic actions of insulin. Am. J. Physiol. 1994;267:E187–E202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- 87.Jahn L.A., Hartline L., Rao N., Logan B., Kim J.J., Aylor K., Gan L.M., Westergren H.U., Barrett E.J. Insulin Enhances Endothelial Function Throughout the Arterial Tree in Healthy But Not Metabolic Syndrome Subjects. J. Clin. Endocrinol. Metab. 2016;101:1198–1206. doi: 10.1210/jc.2015-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Montagnani M., Ravichandran L.V., Chen H., Esposito D.L., Quon M.J. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol. Endocrinol. 2002;16:1931–1942. doi: 10.1210/me.2002-0074. [DOI] [PubMed] [Google Scholar]
- 89.Andreozzi F., Laratta E., Procopio C., Hribal M.L., Sciacqua A., Perticone M., Sesti G. Interleukin-6 impairs the insulin signaling pathway, promoting production of nitric oxide in human umbilical vein endothelial cells. Mol. Cell. Biol. 2007;27:2372–2383. doi: 10.1128/MCB.01340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fleming I., Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 91.Abe H., Yamada N., Kamata K., Kuwaki T., Shimada M., Osuga J., Shionoiri F., Yahagi N., Kadowaki T., Tamemoto H., et al. Hypertension, hypertriglyceridemia, and impaired endothelium-dependent vascular relaxation in mice lacking insulin receptor substrate-1. J. Clin. Investig. 1998;101:1784–1788. doi: 10.1172/JCI1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang C., Li G., Dong H., Sun S., Chen H., Luo D., Sun L., Li X., Chen Z., Yang H., et al. Arg(9)(7)(2) insulin receptor substrate-1 inhibits endothelial nitric oxide synthase expression in human endothelial cells by upregulating microRNA-155. Int. J. Mol. Med. 2015;36:239–248. doi: 10.3892/ijmm.2015.2192. [DOI] [PubMed] [Google Scholar]
- 93.Duplain H., Burcelin R., Sartori C., Cook S., Egli M., Lepori M., Vollenweider P., Pedrazzini T., Nicod P., Thorens B., et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–345. doi: 10.1161/01.CIR.104.3.342. [DOI] [PubMed] [Google Scholar]
- 94.Nigro C., Raciti G.A., Leone A., Fleming T.H., Longo M., Prevenzano I., Fiory F., Mirra P., D'Esposito V., Ulianich L., et al. Methylglyoxal impairs endothelial insulin sensitivity both in vitro and in vivo. Diabetologia. 2014;57:1485–1494. doi: 10.1007/s00125-014-3243-7. [DOI] [PubMed] [Google Scholar]
- 95.Raoch V., Rodriguez-Pascual F., Lopez-Martinez V., Medrano-Andres D., Rodriguez-Puyol M., Lamas S., Rodríguez-Puyol D., López-Ongil S. Nitric oxide decreases the expression of endothelin-converting enzyme-1 through mRNA destabilization. Arterioscler. Thromb. Vasc. Biol. 2011;31:2577–2585. doi: 10.1161/ATVBAHA.111.232025. [DOI] [PubMed] [Google Scholar]
- 96.Liu H., Zhang N., Tian D. MiR-30b is involved in methylglyoxal-induced epithelial-mesenchymal transition of peritoneal mesothelial cells in rats. Cell. Mol. Biol. Lett. 2014;19:315–329. doi: 10.2478/s11658-014-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li S.S., Wu Y., Jin X., Jiang C. The SUR2B subunit of rat vascular KATP channel is targeted by miR-9a-3p induced by prolonged exposure to methylglyoxal. Am. J. Physiol. Cell. Physiol. 2015;308:C139–C145. doi: 10.1152/ajpcell.00311.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Palsamy P., Bidasee K.R., Ayaki M., Augusteyn R.C., Chan J.Y., Shinohara T. Methylglyoxal induces endoplasmic reticulum stress and DNA demethylation in the Keap1 promoter of human lens epithelial cells and age-related cataracts. Free. Radic. Biol. Med. 2014;72:134–148. doi: 10.1016/j.freeradbiomed.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El-Osta A., Brasacchio D., Yao D., Pocai A., Jones P.L., Roeder R.G., Cooper M.E., Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mir A.R., uddin M., Alam K., Ali A. Methylglyoxal mediated conformational changes in histone H2A-generation of carboxyethylated advanced glycation end products. Int. J. Biol. Macromol. 2014;69:260–266. doi: 10.1016/j.ijbiomac.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 101.Mirra P., Nigro C., Prevenzano I., Procopio T., Leone A., Raciti G.A., Andreozzi F., Longo M., Fiory F., Beguinot F., et al. The role of miR-190a in methylglyoxal-induced insulin resistance in endothelial cells. Biochim. Biophys. Acta. 2016;1863:440–449. doi: 10.1016/j.bbadis.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 102.Berlanga J., Cibrian D., Guillen I., Freyre F., Alba J.S., Lopez-Saura P., Merino N., Aldama A., Quintela A.M., Triana M.E., et al. Methylglyoxal administration induces diabetes-like microvascular changes and perturbs the healing process of cutaneous wounds. Clin. Sci. 2005;109:83–95. doi: 10.1042/CS20050026. [DOI] [PubMed] [Google Scholar]
- 103.Masania J., Malczewska-Malec M., Razny U., Goralska J., Zdzienicka A., Kiec-Wilk B., Gruca A., Stancel-Mozwillo J., Dembinska-Kiec A., Rabbani N., et al. Dicarbonyl stress in clinical obesity. Glycoconj. J. 2016;33:581–589. doi: 10.1007/s10719-016-9692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McLellan A.C., Thornalley P.J., Benn J., Sonksen P.H. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin. Sci. 1994;87:21–29. doi: 10.1042/cs0870021. [DOI] [PubMed] [Google Scholar]
- 105.Bierhaus A., Fleming T., Stoyanov S., Leffler A., Babes A., Neacsu C., Sauer S.K., Eberhardt M., Schnölzer M., Lasitschka F., et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat. Med. 2012;18:926–933. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- 106.Giacco F., Du X., D’Agati V.D., Milne R., Sui G., Geoffrion M., Brownlee M. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes. 2014;63:291–299. doi: 10.2337/db13-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Skapare E., Konrade I., Liepinsh E., Strele I., Makrecka M., Bierhaus A., Lejnieks A., Pirags V., Dambrova M. Association of reduced glyoxalase 1 activity and painful peripheral diabetic neuropathy in type 1 and 2 diabetes mellitus patients. J. Diabetes Complicat. 2013;27:262–267. doi: 10.1016/j.jdiacomp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 108.Brouwers O., Niessen P.M., Ferreira I., Miyata T., Scheffer P.G., Teerlink T., Schrauwen P., Brownlee M., Stehouwer C.D., Schalkwijk C.G. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011;286:1374–1380. doi: 10.1074/jbc.M110.144097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tikellis C., Pickering R.J., Tsorotes D., Huet O., Cooper M.E., Jandeleit-Dahm K., Thomas M.C. Dicarbonyl stress in the absence of hyperglycemia increases endothelial inflammation and atherogenesis similar to that observed in diabetes. Diabetes. 2014;63:3915–3925. doi: 10.2337/db13-0932. [DOI] [PubMed] [Google Scholar]
- 110.Kumagai T., Nangaku M., Kojima I., Nagai R., Ingelfinger J.R., Miyata T., Fujita T., Inagi R. Glyoxalase I overexpression ameliorates renal ischemia-reperfusion injury in rats. Am. J. Physiol. Renal. Physiol. 2009;296:F912–F921. doi: 10.1152/ajprenal.90575.2008. [DOI] [PubMed] [Google Scholar]
- 111.Jack M.M., Ryals J.M., Wright D.E. Protection from diabetes-induced peripheral sensory neuropathy—A role for elevated glyoxalase I? Exp. Neurol. 2012;234:62–69. doi: 10.1016/j.expneurol.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vulesevic B., McNeill B., Giacco F., Maeda K., Blackburn N.J., Brownlee M., Milne R.W., Suuronen E.J. Methylglyoxal-Induced Endothelial Cell Loss and Inflammation Contribute to the Development of Diabetic Cardiomyopathy. Diabetes. 2016;65:1699–1713. doi: 10.2337/db15-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]