Abstract
This report summarizes phase 1 studies that evaluated pharmacokinetic interactions between the novel triazole antifungal agent isavuconazole and the immunosuppressants cyclosporine, mycophenolic acid, prednisolone, sirolimus, and tacrolimus in healthy adults. Healthy subjects received single oral doses of cyclosporine (300 mg; n = 24), mycophenolate mofetil (1000 mg; n = 24), prednisone (20 mg; n = 21), sirolimus (2 mg; n = 22), and tacrolimus (5 mg; n = 24) in the presence and absence of clinical doses of oral isavuconazole (200 mg 3 times daily for 2 days; 200 mg once daily thereafter). Coadministration with isavuconazole increased the area under the concentration‐time curves (AUC0–∞) of tacrolimus, sirolimus, and cyclosporine by 125%, 84%, and 29%, respectively, and the AUCs of mycophenolic acid and prednisolone by 35% and 8%, respectively. Maximum concentrations (Cmax) of tacrolimus, sirolimus, and cyclosporine were 42%, 65%, and 6% higher, respectively; Cmax of mycophenolic acid and prednisolone were 11% and 4% lower, respectively. Isavuconazole pharmacokinetics were mostly unaffected by the immunosuppressants. Two subjects experienced elevated creatinine levels in the cyclosporine study; most adverse events were not considered to be of clinical concern. These results indicate that isavuconazole is an inhibitor of cyclosporine, mycophenolic acid, sirolimus, and tacrolimus metabolism.
Keywords: cyclosporine, isavuconazole, mycophenolate mofetil, prednisolone, sirolimus, tacrolimus
Immunosuppressive therapy is required by transplant patients to prevent graft‐vs‐host disease and graft rejection. As a result of their immunocompromised condition, transplant patients are at increased risk of invasive fungal infections,1, 2 which frequently necessitates the use of antifungal agents as part of their management, particularly in a prophylactic setting.1 However, drug‐drug interactions between immunosuppressive agents and triazole antifungals may lead to changes in the exposure of the immunosuppressive agents, which can complicate transplant patient management.
Cyclosporine, mycophenolate mofetil (MMF), prednisone, sirolimus, and tacrolimus are immunosuppressive agents in clinical use for the management of transplant patients. Cyclosporine, prednisolone (the active metabolite of prednisone), sirolimus, and tacrolimus are substrates of cytochrome P450 (CYP) 3A4.3, 4, 5 Previous studies indicate that the triazole antifungal agents fluconazole, voriconazole, itraconazole, and posaconazole are inhibitors of CYP3A4,6 and all have been shown to inhibit the metabolism of cyclosporine, sirolimus, and tacrolimus, while voriconazole also inhibits prednisolone metabolism (see VFEND® package insert). By contrast, mycophenolic acid (MPA; the active agent of MMF) is metabolized via the uridine diphosphate glucuronosyltransferase (UGT) pathway.3 Drug interactions between the triazoles and MPA have not been well studied in clinical trials; fluconazole is a known inhibitor of UGT,6 and isavuconazole is an inhibitor of UGT (data on file), whereas coadministration with voriconazole does not affect the metabolism of MPA (see VFEND package insert).
On the basis of the results of phase 3 clinical trials,7, 8 isavuconazonium sulfate, the prodrug of the broad‐spectrum triazole antifungal agent isavuconazole, was approved in 2015 by the US Food and Drug Administration for the treatment of adults with invasive aspergillosis and with invasive mucormycosis, and by the European Medicines Agency for the treatment of adults with invasive aspergillosis and with mucormycosis when amphotericin B is inappropriate. Isavuconazole is a sensitive substrate and moderate inhibitor of CYP3A4.9 In human liver microsomes expressing CYP3A4 in vitro, 66.2% of isavuconazole was metabolized (data on file). The inhibitory constants (Ki) of isavuconazole with the CYP3A4 substrates midazolam and testosterone in human liver microsomes were 0.62 and 1.93, respectively. The UGT pathway is also involved in isavuconazole's metabolism (manuscript in preparation), and isavuconazole is a mild inhibitor of UGT (in human liver microsomes, IC50 for 17β‐estradiol 3‐glucuronidation [UGT1A1], 9.0 μmol/L; propofol glucuronidation [UGT1A9], 19 μmol/L; morphine 3‐glucuronidation [UGT2B7], 44 μmol/L; data on file). With recommended clinical dosing (200 mg 3 times daily for 2 days, then 200 mg daily), the maximum plasma concentrations observed are typically <7 μg/mL (data on file), and so values of IC50 or Ki ≤16 μmol/L in vitro may suggest potential for drug‐drug interactions in vivo (isavuconazole molecular weight 437.47 g/mol). This report summarizes 5 clinical studies conducted in healthy subjects to assess the pharmacokinetic (PK) interactions between isavuconazole and the CYP3A4 substrates cyclosporine, prednisolone, sirolimus, and tacrolimus, and the UGT substrate MPA.
Methods
Study Design
All clinical study protocols were approved by the Institutional Review Board for each study site (cyclosporine, sirolimus, and tacrolimus studies, Independent Investigational Review Board, Inc., Plantation, Florida; mycophenolate mofetil and prednisone studies, Aspire IRB, LLC, Santee, California). The studies were conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonisation guidelines, and applicable laws and regulations. Signed Institutional Review Board–approved written informed consent was obtained from all subjects prior to any study‐related procedures.
The studies were phase 1 single‐center open‐label drug‐interaction trials conducted in healthy subjects to evaluate effects of isavuconazole (administered as isavuconazonium sulfate; CRESEMBA® oral capsules, Astellas Pharma US, Inc., Northbrook, Illinois) on the PK of coadministered cyclosporine (NEORAL® oral capsules, Novartis Pharmaceuticals Corp, East Hanover, New Jersey; trial conducted November 2011 to January 2012 at Covance, Madison, Wisconsin; NCT01494597), MMF (CELLCEPT® oral tablets, Genentech USA, Inc., South San Francisco, California; trial conducted March to April 2012 at PAREXEL International, LLC, Glendale, California; NCT01711489), prednisolone (generic oral capsules of prodrug prednisone; trial conducted February to March 2012 at PAREXEL International, LLC, Glendale, California; NCT01711827), sirolimus (RAPAMUNE® oral tablets, Pfizer Inc., Philadelphia, Pennsylvania; trial conducted December 2011 to January 2012 at Covance, Dallas, Texas; NCT01809860), and tacrolimus (PROGRAF® oral capsules, Astellas Pharma US, Inc., Northbrook, Illinois; trial conducted December 2011 to January 2012 at Covance, Madison, Wisconsin; NCT01535547).
Healthy, medication‐free adult male and female subjects, aged 18‐55 years old, who weighed ≥45 kg, and with a body mass index of 18‐32 kg/m2 were included in these studies.
Dosing and Sampling Schedules
Dosing information is expressed as the isavuconazole equivalent of the prodrug: oral capsules each contained isavuconazonium sulfate 186 mg, equivalent to isavuconazole 100 mg. The clinically targeted oral dose of isavuconazonium sulfate 372 mg (equivalent to isavuconazole 200 mg) 3 times a day loading dose (TID), followed by 372 mg once daily (QD) was used in the studies. Only the active metabolite isavuconazole is referred to hereafter.
Cyclosporine
Subjects were screened between days –28 and –2 and checked in at the study center on day –1, where they remained until day 5 and from days 10 to 19. Subjects returned to the study center on day 25 (±2 days) for a follow‐up assessment.
On day 1, subjects received a single oral dose of cyclosporine 300 mg. Following a 10‐day washout, subjects received an oral loading dose of isavuconazole 200 mg TID on days 11 and 12, followed by 200 mg QD on days 13 to 18 (Figure 1A). A single oral dose of cyclosporine 300 mg was also administered to subjects on day 15. Subjects were administered both doses of cyclosporine while fasting (at least 10 hours prior to dosing and 4 hours after dosing). Isavuconazole was administered immediately following cyclosporine on day 15.
Figure 1.
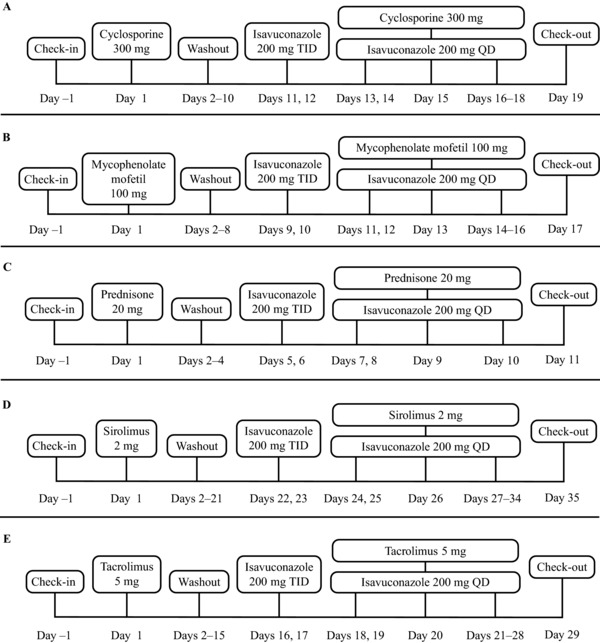
Study dosing schedules. Isavuconazole 200 mg was administered as isavuconazonium sulfate 372 mg. QD, once daily; TID, 3 times a day.
Blood samples were collected for PK analysis of cyclosporine on days 1 and 15 at predose and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, 36, 48, 72, and 96 hours postdose. Samples were collected for PK analysis of isavuconazole on day 14 at predose and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, and 16 hours postdose as well as on day 15 at predose and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, and 36 hours postdose.
Mycophenolate Mofetil
After screening (day –28 to –2), subjects checked in at the study center on day –1, where they remained until day 5 and again from day 8 until day 17. Subjects returned to the study center on day 24 (±2 days) for a follow‐up assessment.
On day 1 of the study subjects received a single oral dose of MMF 1 g, followed by a 7‐day washout period. Subjects then received an oral loading dose of isavuconazole 200 mg TID on days 9 and 10, followed by 200 mg QD on days 11 to 16 (Figure 1B). On day 13 subjects received a single oral dose of MMF 1 g concurrently with isavuconazole. Subjects were administered both doses of MMF while fasting (at least 10 hours prior to dosing and 4 hours after dosing). Isavuconazole was administered immediately following MMF on day 13.
Blood samples were collected for PK analysis of MPA and its metabolite, mycophenolic acid phenyl glucuronide (MPAG), on days 1 and 13 at predose and at 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, 24, 48, 72, and 96 hours postdose. Samples were also collected for PK analysis of isavuconazole on days 12 and 13 at predose and at 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, and 24 hours postdose.
Prednisolone
Subjects were screened from day –28 to –2 and checked in at the study center on day –1, where they remained until day 11. A follow‐up visit was conducted at the study center on day 18 (±2 days).
On day 1, subjects received a single oral dose of prednisone 20 mg (prodrug of prednisolone). Following a 4‐day washout period, subjects received an oral loading dose of isavuconazole 200 mg TID on days 5 and 6, followed by 200 mg QD on days 7 to 10 (Figure 1C). On day 9, subjects received a single oral dose of prednisone 20 mg with isavuconazole. Subjects were administered both doses of prednisone while fasting (at least 10 hours prior to dosing and 4 hours after dosing). Isavuconazole was administered immediately following prednisone on day 9.
Blood samples were collected for PK analysis of prednisone and its active metabolite prednisolone on days 1 and 9 at predose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, 24, and 48 hours postdose. Samples were also collected for PK analysis of isavuconazole on days 8 and 9 at predose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, and 24 hours postdose.
Sirolimus
Following screening (day –28 to day –2), subjects checked in at the study center on day –1, where they remained until day 4 and from day 21 to day 35. Subjects returned for a follow‐up assessment on day 41 (±2 days).
On day 1 subjects received a single dose of oral sirolimus 2 mg. After a 21‐day washout, subjects received a loading dose of oral isavuconazole 200 mg TID on days 22 and 23, followed by 200 mg QD on days 24 to 34 (Figure 1D). On day 26, subjects received a single dose of oral sirolimus 2 mg in addition to isavuconazole. Subjects were administered both doses of sirolimus while fasting (at least 10 hours prior to dosing and 4 hours after dosing). Isavuconazole was administered immediately following sirolimus on day 26.
Blood samples were collected for PK analysis of sirolimus on days 1 and 26 at predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, 48, 72, 96, 120, 144, 168, 192, and 216 hours postdose. Samples were collected for PK analysis of isavuconazole on day 25 at predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 16 hours postdose as well as on day 26 at predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, and 36 hours postdose.
Tacrolimus
Subjects were screened between days –28 and –2 and checked in at the study center on day –1. Following check‐in, subjects remained in the study center until day 5 and between days 15 and 29. Subjects returned to the study center on day 35 (±2 days) for a follow‐up assessment.
On day 1, subjects received a single oral dose of tacrolimus 5 mg. Following a 15‐day washout, subjects received an oral loading dose of isavuconazole 200 mg TID on days 16 and 17, followed by 200 mg QD on days 18 to 28 (Figure 1E). A single oral dose of tacrolimus 5 mg was also administered to subjects on day 20. Subjects were administered both doses of tacrolimus while fasting (at least 10 hours prior to dosing and 4 hours after dosing). Isavuconazole was administered immediately following tacrolimus on day 20.
Blood samples were collected for PK analysis of tacrolimus on days 1 and 20 at predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, 36, 48, 72, 96, 120, 144, 168, 192, and 216 hours postdose. Samples were collected for PK analysis of isavuconazole on day 19 at predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, and 16 hours postdose as well as on day 20 at predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, and 36 hours postdose.
Pharmacokinetic Assessments
Plasma or whole‐blood concentrations of all analytes were measured by liquid chromatography–mass spectrometry/mass spectrometry (see Supplementary Data S1 for methods). The primary PK variables for the immunosuppressive agents were area under the concentration‐time curve (AUC) from time 0 to infinity (AUC0–∞), AUC from time of dosing to time of last measurable concentration (AUClast), and maximum drug concentration (Cmax). Secondary variables included time to Cmax (tmax), apparent volume of distribution (Vz/F), apparent body clearance after dosing (CL/F), and elimination half‐life (t1/2). Because the reversible conversion of the prodrug prednisone to prednisolone results in wide interindividual variability in Vz/F and CL/F, those parameters were not measured for prednisolone. Cyclosporine, sirolimus, and tacrolimus parameters were measured in whole blood. Prednisone, prednisolone, MPA, and MPAG were measured in plasma. For isavuconazole, area under the plasma concentration‐time curve for a dosing interval (AUCτ), Cmax, and tmax were calculated.
Safety Assessments
Treatment‐emergent adverse events (TEAEs) were assessed throughout the studies. Other safety assessments included vital‐sign measurements, 12‐lead electrocardiograms, clinical laboratory testing (hematology, chemistry, and urinalysis), and physical examinations.
Statistics
Descriptive statistics were used to summarize demographics, baseline characteristics, and TEAEs for all patients who received ≥1 dose of study drug. Pharmacokinetics were assessed in all subjects who received ≥1 dose of study drug and with PK data sufficient for calculation of ≥1 primary PK parameter. Levels of analyte below the level of quantification were entered as 0 for calculations. For PK assessments, log‐transformed AUC and Cmax values were analyzed using a mixed‐effects model with treatment as a fixed effect and subject as a random effect. Treatment was defined as coadministration with an immunosuppressive agent plus isavuconazole and administration of the immunosuppressive agent alone. Results are presented as 90% confidence intervals (CIs) constructed around the geometric least‐squares mean ratios of PK parameters measured during dosing with an immunosuppressive agent plus isavuconazole vs dosing with the immunosuppressive agent alone. Pharmacokinetics were assessed using noncompartmental analysis with Phoenix® WinNonlin® version 5.2.1 or higher (Certara USA, Inc., Princeton, New Jersey). All data processing, summarization, and analyses were performed using SAS® version 9.1 (Statistical Analysis Software, Cary, North Carolina). Statistical analysis of isavuconazole and prednisone PK was not prespecified or performed in the prednisone study.
Results
Pharmacokinetics
Cyclosporine
Twenty‐four healthy subjects were enrolled and 19 completed the cyclosporine study. Demographics and baseline characteristics are shown in Table 1. Mean AUC0–∞, AUClast, and Cmax values for cyclosporine were 29%, 29%, and 6% higher, respectively, in the presence vs absence of isavuconazole (Tables 2 and 3; Figure 2). The PK of isavuconazole is shown in Table 4. The mean Cmax of isavuconazole was 30% higher in the presence vs absence of cyclosporine, whereas the mean AUCτ was 3% higher.
Table 1.
Demographics and Baseline Characteristics
| Parameter | Cyclosporine (n = 24) | Mycophenolate Mofetil (n = 24) | Prednisone (n = 21) | Sirolimus (n = 22) | Tacrolimus (n = 24) |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 19 (79.2) | 15 (62.5) | 14 (66.7) | 13 (59.1) | 15 (62.5) |
| Female | 5 (20.8) | 9 (37.5) | 7 (33.3) | 9 (40.9) | 9 (37.5) |
| Race, n (%) | |||||
| White | 19 (79.2) | 13 (54.2) | 11 (52.4) | 12 (54.5) | 16 (66.7) |
| Black or African American | 4 (16.7) | 10 (41.7) | 10 (47.6) | 9 (40.9) | 5 (20.8) |
| Asian | 1 (4.2) | 0 | 0 | 0 | 1 (4.2) |
| Othera | 0 | 1 (4.2) | 0 | 1 (4.5) | 2 (8.3) |
| Ethnicity, n (%) | |||||
| Not Hispanic or Latino | 22 (91.7) | 20 (83.3) | 16 (76.2) | 14 (63.6) | 23 (95.8) |
| Age, years, mean (SD) | 32.0 (9.9) | 35.0 (9.9) | 31.0 (9.7) | 36.6 (8.8) | 35 (11.4) |
| Weight, kg, mean (SD) | 75.4 (15.4) | 75.1 (13.3) | 77.7 (14.5) | 75.2 (14.6) | 75.2 (14.5) |
| Body mass index, kg/m2, mean (SD) | 24.7 (3.5) | 25.1 (3.8) | 26.0 (3.7) | 25.7 (3.3) | 25.6 (3.0) |
SD, standard deviation.
“Other” category includes white/Native American, white/American Indian or Alaskan Native, and African American or American Indian.
Table 2.
Pharmacokinetics of Immunosuppressive Agents
| Cyclosporine | Mycophenolic Acid | Prednisolone | Sirolimus | Tacrolimus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parametera | CSA Alone (n = 24) | CSA + Isavuconazole (n = 19) | MMF Alone (n = 24) | MMF + Isavuconazole (n = 22) | PRE Alone (n = 21) | PRE + Isavuconazole (n = 20) | SIR Alone (n = 22) | SIR + Isavuconazole (n = 21) | TAC Alone (n = 24) | TAC + Isavuconazole (n = 21) |
| AUC0–∞, ng·h/mL | 7453 (1579) | 9603 (1933) | 67 310 (18 533) | 93 477 (28 546) | 1653 (227) | 1783 (288) | 195.6 (72.2) | 346.2 (75.9) | 312.7 (120.8) | 695.1 (261.4) |
| AUClast, ng·h/mL | 7043 (1512) | 9079 (1830) | 64 965 (18 607) | 87 789 (28 520) | 1645 (223) | 1775 (282) | 152.4 (62.4) | 303.2 (67.6) | 304.4 (119.3) | 680.0 (250.8) |
| Cmax, ng/mL | 1340 (260) | 1431 (354) | 27 193 (8208) | 24 592 (9097) | 294.9 (49.7) | 282.3 (47.5) | 5.1 (1.6) | 8.5 (2.7) | 26.7 (8.3) | 37.1 (9.6) |
| tmax, hours | 1.5 (1.0‐3.0) | 1.5 (1.0‐3.0) | 0.5 (0.5‐1.5) | 0.5 (0.5‐2.0) | 1.5 (0.5‐4.0) | 2.0 (0.5‐5.0) | 2.5 (1.5‐10.0) | 2.0 (1.5–6.0) | 1.5 (0.8–3.0) | 3.0 (1.5–5.0) |
| t1/2, hours | 37.7 (10.0) | 38.1 (12.0) | 12.6 (4.0) | 19.3 (5.5) | 2.9 (0.4) | 2.7 (0.3) | 74.8 (19.0) | 78.5 (10.0) | 40.0 (9.8) | 37.5 (6.0) |
| CL/F, L/h | 41.9 (8.6) | 33.3 (11.5) | 11.5 (3.4) | 8.5 (2.7) | —b | —b | 11.3 (3.4) | 6.1 (1.4) | 18.4 (7.1) | 8.0 (2.4) |
AUC, area under the concentration‐time curve; CL/F, clearance; Cmax, maximum concentration; CSA, cyclosporine; MMF, mycophenolate mofetil; PRE, prednisone; SIR, sirolimus; TAC, tacrolimus; tmax, time to maximum concentration; t1/2, half‐life.
AUC0–∞, AUClast, Cmax, t1/2, CL/F, and Vz/F values are expressed as arithmetic mean (standard deviation); tmax is expressed as median (range).
Not calculated.
Table 3.
Statistical Analysis of the Effect of Isavuconazole on the Pharmacokinetics of Immunosuppressive Agents
| Geometric Least‐Squares Mean Ratio, % (90%CI) | |||||
|---|---|---|---|---|---|
| Parameter | Cyclosporine | Mycophenolic Acid | Prednisolone | Sirolimus | Tacrolimus |
| AUC0–∞ | 129 (115, 144) | 135 (127, 145) | 108 (102, 114) | 184 (159, 213) | 225 (191, 266) |
| AUClast | 129 (115, 144) | 132 (124, 141) | 108 (102, 114) | 208 (181, 239) | 227 (192, 268) |
| Cmax | 106 (95, 119) | 89 (76, 103) | 96 (90, 102) | 165 (141, 192) | 142 (122, 164) |
AUC, area under the concentration‐time curve; CI, confidence interval; Cmax, maximum concentration.
Figure 2.
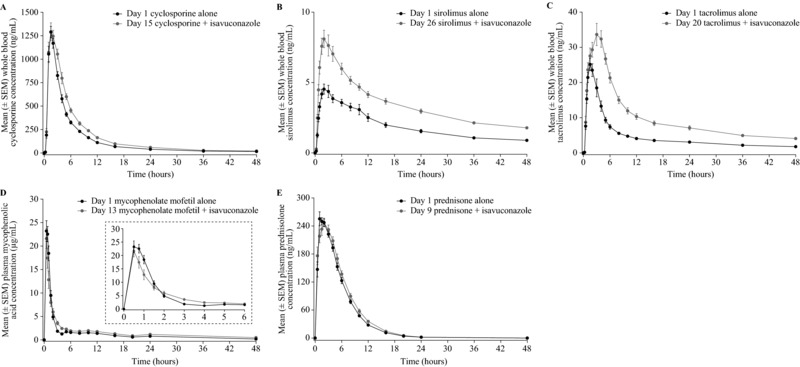
Mean whole‐blood/plasma concentration‐time profiles up to 48 hours for cyclosporine (A), sirolimus (B), tacrolimus (C), mycophenolic acid (D; inset shows expanded 0 to 6 hours time interval), and prednisolone (E) in the presence and absence of isavuconazole. SEM, standard error of the mean.
Table 4.
Pharmacokinetics of Isavuconazole
| Cyclosporine | Mycophenolate Mofetil | Prednisone | Sirolimus | Tacrolimus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Isavuconazole Alone (n = 21) | CSA + Isavuconazole (n = 19) | Isavuconazole Alone (n = 22) | MMF + Isavuconazole (n = 22) | Isavuconazole Alone (n = 20) | PRE + Isavuconazole (n = 20) | Isavuconazole Alone (n = 21) | SIR + Isavuconazole (n = 21) | Isavuconazole Alone (n = 21) | TAC + Isavuconazole (n = 21) |
| AUCτ, μg·h/mLa | 107.7 (31.2) | 109.3 (29.2) | 101.4 (37.6) | 100.5 (32.3) | 88.2 (37.9) | 98.8 (36.6) | 92.0 (21.0) | 100.4 (24.2) | 89.8 (33.8) | 100.4 (35.5) |
| Cmax, μg/mLa | 5.7 (1.9) | 7.3 (1.8) | 6.5 (2.1) | 6.7 (2.0) | 5.6 (1.9) | 6.7 (2.4) | 6.2 (1.4) | 6.4 (1.5) | 4.9 (1.6) | 6.10 (1.7) |
| tmax, hours | 3.0 (0.5‐8.0) | 4.0 (3.0‐5.0) | 3.0 (1.0‐4.0) | 3.0 (1.5‐5.0) | 2.0 (1.5‐3.0) | 3.0 (1.5‐5.0) | 2.0 (1.5‐3.0) | 3.0 (2.0‐4.0) | 3.0 (0.3‐8.0) | 3.0 (1.5‐8.0) |
| Geometric Least‐Squares Mean, % (90%CI) | ||||||||||
| AUCτ | 103 (100, 106) | 101 (98, 103) | —b | 111 (109, 114) | 112 (110, 115) | |||||
| Cmax | 130 (121, 139) | 104 (100, 107) | —b | 104 (100, 108) | 126 (118, 134) | |||||
AUC, area under the concentration‐time curve; Cmax, maximum concentration; CI, confidence interval; CSA, cyclosporine; MMF, mycophenolate mofetil; PRE, prednisone; SIR, sirolimus; TAC, tacrolimus; tmax, time to maximum concentration.
AUCτ and Cmax values are expressed as arithmetic mean (standard deviation); tmax is expressed as median (range).
Not calculated.
Mycophenolic Acid
Twenty‐four healthy subjects were enrolled and 21 completed the MMF study. Demographics and baseline characteristics are shown in Table 1. Mean AUC0–∞ and AUClast values for MPA were 35% and 32% higher, respectively, whereas Cmax was 11% lower, in the presence vs absence of isavuconazole (Tables 2 and 3; Figure 2). AUC0–∞, AUClast, and Cmax values for MPAG PK were 24%, 27%, and 32% lower, respectively, in the presence vs absence of isavuconazole (Supplementary Tables S1, S2). The PK of isavuconazole is shown in Table 4. The mean Cmax of isavuconazole was increased by 4% by coadministration with MMF, whereas the mean AUCτ was unchanged.
Prednisolone
Twenty‐one healthy subjects were enrolled and 20 completed the prednisone study. Demographics and baseline characteristics are shown in Table 1. Mean AUC0–∞ and AUClast values for prednisolone were 8% higher, whereas Cmax was 4% lower, in the presence vs absence of isavuconazole (Tables 2 and 3; Figure 2). The PK of prednisone is shown in Supplementary Table S1. The PK of isavuconazole is shown in Table 4. The Cmax of isavuconazole was approximately 26% higher in the presence versus absence of prednisilone, whereas AUCτ was virtually unchanged (mean differences not calculated).
Sirolimus
Twenty‐two healthy subjects were enrolled and 21 completed the sirolimus study. Demographics and baseline characteristics are shown in Table 1. Mean AUC0–∞, AUClast, and Cmax values for sirolimus were 84%, 108%, and 65% higher, respectively, in the presence vs absence of isavuconazole (Tables 2 and 3; Figure 2). The PK of isavuconazole is shown in Table 4. The mean AUCτ and Cmax of isavuconazole were 11% and 4% higher in the presence of sirolimus.
Tacrolimus
Twenty‐four healthy subjects were enrolled and 20 completed the tacrolimus study. Demographics and baseline characteristics are shown in Table 1. Mean AUC0–∞, AUClast, and Cmax values for tacrolimus were 125%, 127%, and 42% higher, respectively, in the presence vs absence of isavuconazole (Tables 2 and 3; Figure 2). The PK of isavuconazole is shown in Table 4. The mean AUCτ and Cmax of isavuconazole were 12% and 26% higher in the presence of tacrolimus.
Safety
No serious TEAEs occurred during any of the studies. The most common TEAEs in the cyclosporine study were headache (n = 8), nausea (n = 8), feeling hot (n = 6), dizziness (n = 4), diarrhea (n = 4), and abdominal discomfort (n = 4) (Supplementary Table S3). Two subjects discontinued due to TEAEs of increased creatinine during isavuconazole‐only administration, which was considered to be possibly related to treatment by the study investigator. In 1 subject, blood creatinine increased from 0.9 mg/dL on day –1 to 1.4 mg/dL on day 15. The second subject had high levels of creatinine at baseline (1.3 mg/dL), which increased to 1.4 mg/dL by day 14. One subject discontinued due to TEAEs of increased blood pressure and heart rate during cyclosporine‐only administration, which was also considered as possibly related to study treatment. Two additional subjects discontinued during cyclosporine‐only administration due to TEAEs that were considered unrelated to cyclosporine.
The most common TEAEs in the MMF study were hot flush (n = 4) and headache (n = 3) (Supplementary Table S4).
In the prednisone study the most common TEAEs were headache (n = 4) and diarrhea (n = 4) (Supplementary Table S5). One subject discontinued the study early due to a TEAE of intermittent diarrhea of moderate intensity during isavuconazole alone administration, which was considered to be probably related to treatment.
The most common TEAEs in the sirolimus study were feeling hot (n = 5) and somnolence (n = 4) (Supplementary Table S6). No TEAEs resulted in discontinuation during either study.
Last, the most common TEAEs in the tacrolimus study were feeling hot (n = 10), headache (n = 6), and dizziness (n = 5) (Supplementary Table S7). Four subjects discontinued treatment due to TEAEs: 3 of those subjects experienced mild TEAEs during tacrolimus alone, which were considered unrelated to the study treatments, and 1 subject experienced sensations of warmth, intermittent flushing, and tingling of the lips during tacrolimus plus isavuconazole coadministration, which were considered probably related to treatment.
Discussion
Five phase 1 studies were conducted in healthy volunteers to evaluate the PK and safety effects of isavuconazole coadministration with the immunosuppressants cyclosporine, MMF, prednisone, sirolimus, and tacrolimus. Coadministration of the clinically targeted dose of isavuconazole with the immunosuppressants revealed a varied drug‐interaction profile that ranged from mild to moderate inhibition in healthy adults.
All triazoles have been shown to inhibit the metabolism of cyclosporine, sirolimus, and tacrolimus.6 Fluconazole is a moderate inhibitor of CYP3A4 and has been found to increase cyclosporine exposure by 2‐ to 3‐fold when the 2 agents are coadministered, compared with cyclosporine alone.6 In addition, blood levels of sirolimus are reported to be approximately 3‐ to 5‐fold higher in the presence vs the absence of fluconazole,6 and dose reductions of ∼50% for tacrolimus may be required for the safe coadministration of fluconazole.10, 11 Monitoring of patients and possible dose adjustment of cyclosporine, sirolimus, and tacrolimus are recommended when these immunosuppressants are coadministered with fluconazole (see DIFLUCAN® package insert).
Voriconazole, itraconazole, and posaconazole are strong inhibitors of CYP3A4 and display pronounced interactions with cyclosporine, sirolimus, and tacrolimus (Table 5). For example, the mean AUC of sirolimus was 11‐fold higher during coadministration with oral voriconazole in healthy male subjects, compared with sirolimus alone (VFEND package insert). As a result, labeling information indicates that coadministration of voriconazole and sirolimus is contraindicated. Owing to potential increases in exposure to cyclosporine (Cmax, 30% increase; AUCτ, 3% increase), sirolimus (Cmax, 4% increase; AUCτ, 11% increase), and tacrolimus (Cmax, 26% increase; AUCτ, 12% increase) in patients coadministered with isavuconazole, therapeutic drug monitoring of the immunosuppressants is recommended, and dose adjustments of cyclosporine, sirolimus, and tacrolimus may be necessary.
Table 5.
Interactions Between Triazole Antifungals and the Immunosuppressants Cyclosporine, Sirolimus, and Tacrolimus
| CYP3A4 Substrate | Isavuconazole | Fluconazole | Itraconazole | Posaconazole | Voriconazole |
|---|---|---|---|---|---|
| Cyclosporine | ↑ 1.3a | ↑ 2‐3a ,6 | ↑ 2.7b ,13 | ↑b , c ,14 | ↑ 1.7a ,7 |
| Sirolimus | ↑ 1.8a | ↑ 3‐4.7b ,6 | NA | ↑ 7.9a ,14 | ↑ 11a ,7 |
| Tacrolimus | ↑ 2.3a | NA | ↑ 5.6b ,13 | ↑ 3.6a ,14 | ↑ 3a ,7 |
NA, not applicable.
Fold increases in area under the plasma concentration‐time curve.
Fold increases in trough concentrations.
Increase observed, but fold change not reported.
The UGT pathway is involved in the secondary metabolism of isavuconazole following metabolism by CYP3A4 and CYP3A5, and a number of its metabolites are glucuronidated (manuscript in preparation). Isavuconazole is also a mild inhibitor of UGT enzymes in vitro (data on file), and so the increase in MPA exposure observed in the MMF study is the result of UGT inhibition by isavuconazole. By comparison, coadministration with voriconazole has no significant effect on MPA AUC (VFEND package insert), whereas studies of coadministration of MMF with fluconazole, itraconazole, and posaconazole have not been reported. The principal adverse events associated with MMF are diarrhea, leukopenia, sepsis, vomiting, and a higher frequency of opportunistic infection as well as an increased risk of lymphoma and other malignancies, particularly of the skin (see CELLCEPT package insert). Therefore, it is recommended that patients given MMF with concomitant isavuconazole be monitored for MPA‐related toxicities.
There was little change to the AUC of prednisolone when prednisone and isavuconazole were given together, indicating the absence of a clinically relevant interaction between these 2 agents. Similar findings have been reported for itraconazole and prednisolone coadministration.12 By contrast, voriconazole has demonstrated weak inhibition of prednisolone metabolism. There are no published PK studies of fluconazole or posaconazole coadministration with prednisone.
Coadministration with the immunosuppressive agents in the current studies was associated with minimal changes to the PK of isavuconazole itself. Changes to isavuconazole PK were not considered to be clinically relevant and were within the range identified in population PK studies of isavuconazole.9
In this series of studies there were no serious TEAEs, and few subjects discontinued due to TEAEs. Two subjects in the cyclosporine study experienced elevated creatinine levels that were considered to be possibly related to the study treatments.
In summary, these findings indicate that clinical doses of isavuconazole can be administered together with a number of immunosuppressive agents likely to be used in transplant patients at risk of fungal infections. The degree of interaction between isavuconazole and the immunosuppressive agents under investigation appears to be less than that reported for other triazole antifungal agents. This is also consistent with the moderate CYP3A4 inhibition demonstrated in an accompanying study.9 However, attention to systemic drug levels and possible dose adjustments are likely to be necessary for cyclosporine, sirolimus, and tacrolimus in patients given concomitant isavuconazole to ensure that therapeutic concentrations are maintained and adverse pharmacodynamic effects are avoided. In addition, patients given MMF with isavuconazole should be monitored for potential MPA‐related adverse reactions.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Supporting Information
Supporting Information
Acknowledgments
These studies were funded by Astellas Pharma Global Development, Inc. Isavuconazonium sulfate was codeveloped by Astellas Pharma Global Development, Inc. and Basilea Pharmaceutica International Ltd. Medical writing support was provided by Radhika Bhatia, PhD, and Neil M Thomas, PhD, medical writers at Envision Scientific Solutions, funded by Astellas Pharma Global Development, Inc. The authors are grateful to the investigators and staff who conducted the clinical trials and to the subjects who volunteered for these studies.
Declaration of Conflicting Interests
Isavuconazole was codeveloped by Astellas Pharma Global Development, Inc. and Basilea Pharmaceutica International Ltd. A.H.G. has served on a speaker's bureau and acted as a consultant for Astellas. A.D., C.H., S.A., D.K., C.L., H.P., T.Y., and R.T. are employees of Astellas Pharma Global Development, Inc. D.H. is an employee of PAREXEL who was contracted by Astellas Pharma Global Development, Inc. to perform work related to the study. K.K. is an employee of Astellas Pharma Inc. W.L. and D.M. are employees of Covance Clinical Research who were contracted by Astellas Pharma Global Development, Inc. to perform work related to the study.
References
- 1. Fishman JA. Infections in immunocompromised hosts and organ transplant recipients: essentials. Liver Transpl. 2011;17(3):S34–S37. [DOI] [PubMed] [Google Scholar]
- 2. Pagano L, Akova M, Dimopoulos G, Herbrecht R, Drgona L, Blijlevens N. Risk assessment and prognostic factors for mould‐related diseases in immunocompromised patients. J Antimicrob Chemother. 2011;66(1):i5–i14. [DOI] [PubMed] [Google Scholar]
- 3. de Jonge H, Naesens M, Kuypers DR. New insights into the pharmacokinetics and pharmacodynamics of the calcineurin inhibitors and mycophenolic acid: possible consequences for therapeutic drug monitoring in solid organ transplantation. Ther Drug Monit. 2009;31:416–435. [DOI] [PubMed] [Google Scholar]
- 4. Bergmann TK, Barraclough KA, Lee KJ, Staatz CE. Clinical pharmacokinetics and pharmacodynamics of prednisolone and prednisone in solid organ transplantation. Clin Pharmacokinet. 2012;51:711–741. [DOI] [PubMed] [Google Scholar]
- 5. Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome P‐450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos. 1992;20:753–761. [PubMed] [Google Scholar]
- 6. Nivoix Y, Ubeaud‐Sequier G, Engel P, Leveque D, Herbrecht R. Drug‐drug interactions of triazole antifungal agents in multimorbid patients and implications for patient care. Curr Drug Metab. 2009;10:395–409. [DOI] [PubMed] [Google Scholar]
- 7. Maertens JA, Raad, II , Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised‐controlled, non‐inferiority trial. Lancet. 2016;387:760–769. [DOI] [PubMed] [Google Scholar]
- 8. Marty FM, Ostrosky‐Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single‐arm open‐label trial and case‐control analysis. Lancet Infect Dis. 2016;16:828–837. [DOI] [PubMed] [Google Scholar]
- 9. Townsend R, Dietz AJ, Hale C, et al. Pharmacokinetic evaluation of CYP3A4‐mediated drug‐drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin Pharmacol Drug Dev. 2017;6:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahnke CB, Sutton RM, Venkataramanan R, et al. Tacrolimus dosage requirements after initiation of azole antifungal therapy in pediatric thoracic organ transplantation. Pediatr Transplant. 2003;7:474–478. [DOI] [PubMed] [Google Scholar]
- 11. Manez R, Martin M, Raman D, et al. Fluconazole therapy in transplant recipients receiving FK506. Transplantation. 1994;57:1521–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lebrun‐Vignes B, Archer VC, Diquet B, et al. Effect of itraconazole on the pharmacokinetics of prednisolone and methylprednisolone and cortisol secretion in healthy subjects. Br J Clin Pharmacol. 2001;51:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Supporting Information
Supporting Information


