Abstract
IMPORTANCE
Recent reports have demonstrated a higher incidence of autism spectrum disorder (ASD) and substantially elevated autistic trait burden in individuals with neurofibromatosis type 1 (NF1). However, important discrepancies regarding the distribution of autistic traits, sex predominance, and association between ASD symptoms and attentional problems have emerged, and critical features of the ASD phenotype within NF1 have never been adequately explored. Establishing NF1 as a monogenic cause for ASD has important implications for affected patients and for future research focused on establishing convergent pathogenic mechanisms relevant to the potential treatment targets for ASD.
OBJECTIVE
To characterize the quantitative autistic trait (QAT) burden in a pooled NF1 data set.
DESIGN, SETTING, AND PARTICIPANTS
Anonymized, individual-level primary data were accumulated from 6 tertiary referral centers in the United States, Belgium, United Kingdom, and Australia. A total of 531 individuals recruited from NF1 clinical centers were included in the study.
MAIN OUTCOMES AND MEASURES
Distribution of ASD traits (Social Responsiveness Scale, second edition [SRS-2], with T scores of ≥75 associated with a categorical ASD diagnosis); attention-deficit/hyperactivity disorder (ADHD) traits (4 versions of Conners Rating Scale, with T scores of ≥65 indicating clinically significant ADHD symptoms); ASD symptom structure, latent structure, base rate derived from mixture modeling; and familiality.
RESULTS
Of the 531 patients included in the analysis, 247 were male (46.5%); median age was 11 years (range, 2.5–83.9 years). QAT scores were continuously distributed and pathologically shifted; 13.2%(95%CI, 10.3%–16.1%) of individuals scored within the most severe range (ie, above the first percentile of the general population distribution) in which the male to female ratio was markedly attenuated (1.6:1) relative to idiopathic ASD. Autistic symptoms in this NF1 cohort demonstrated a robust unitary factor structure, with the first principal component explaining 30.9%of the variance in SRS-2 scores, and a strong association with ADHD symptoms (r = 0.61). Within-family correlation for QAT burden (intraclass correlation coefficient, 0.73 in NF1-affected first-degree relatives) exceeded that observed in the general population and ASD family samples.
CONCLUSIONS AND RELEVANCE
This study provides confirmation that the diversity of mutations that give rise to NF1 function as quantitative trait loci for ASD. Moreover, the within-family correlation implicates a high degree of mutational specificity for this associated phenotype. Clinicians should be alerted to the increased frequency of this disabling comorbidity, and the scientific community should be aware of the potential for this monogenic disorder to help elucidate the biological features of idiopathic autism.
Autism spectrum disorder (ASD) is a common neurodevelopmental disorder characterized by impairments in social interaction and communication and the presence of restricted interests and repetitive behaviors.1 Autism spectrum disorder affects 1% to 2% of children worldwide,2 leading to pervasive social challenges and reduced quality of life.3 A major role of genetic factors in the causation of ASD has been supported by genetic epidemiologic studies and has been further strengthened by recent molecular genetic studies4,5 and by the co-occurrence of autistic syndromes in monogenic conditions, such as fragile X syndrome, tuberous sclerosis, and neurofibromatosis type 1 (NF1).
Neurofibromatosis type 1 is an autosomal dominant disorder characterized by multiple café-au-lait spots, skin fold freckling, Lisch nodules, distinctive osseous lesions, and nervous system tumors (eg, neurofibromas and optic pathway gliomas).6 Neurofibromatosis type 1 is caused by a diverse constellation of loss-of-function mutations in the NF1 gene (OMIM 613113) on chromosome 17q11.2, a locus with one of the highest known single-gene mutation rates in the human genome.7 Recently, several single-center studies investigating quantitative autistic trait (QAT) burden in individuals with NF1 have reported elevated autistic symptoms,8–12 and one population-based study13 (N = 109) using in-person diagnostic assessment reported a 25% rate of categorical ASD with a phenotypic profile similar to idiopathic autism and a 20% rate of partial ASD features. However, these studies have reached conflicting conclusions regarding sex predominance, the association between ASD symptoms and attention-deficit/hyperactivity disorder (ADHD), and the distribution of autistic traits in NF1, all of which have potentially critical implications for understanding social disability in this monogenic disorder and how NF1-associated ASD might relate to idiopathic autism syndromes.
To address these issues with adequate statistical power, it was necessary to convene an international consortium of NF1 academic centers (International NF1-ASD Consortium Team [INFACT]) to assemble the largest collection of patients with NF1 for whom autistic traits have been uniformly ascertained. A major goal was to resolve whether the aggregation of ASD in NF1 relates exclusively to a specific subgroup of patients with NF1 or rather results from a pathologic shift of the distribution of ASD traits across the entire NF1 population. This distinction is crucial to understanding the manner in which the diversity of mutations that give rise to NF1 influence ASD symptoms as well as the candidacy of NF1 (OMIM 613113) as a quantitative trait locus for ASD. Another goal was to determine whether the social impairments of a given individual with NF1 are influenced by other common NF1-related neurodevelopmental impairments, namely, intellectual deficits and ADHD.14,15 A third major goal involved investigating symptom structure, that is, whether the aggregation of ASD traits in NF1 is sex specific (as in idiopathic ASD), a function of specific subsets of autistic traits driving the accumulated symptom burden in NF1, or both.
Key Points.
Question
What is the nature of autistic symptomatology in neurofibromatosis type 1 (NF1)?
Findings
In this analysis of pooled, individual-level primary data from 531 individuals, males and females with NF1 exhibited a significant burden of autistic traits and symptoms in a continuous distribution that encompassed the full range of mild (subclinical) to severe (clinical). When NF1 was inherited, there was a high degree of within-family association for the severity of autistic traits.
Meaning
Autistic symptoms represent a variable, mutation-specific component of the neurodevelopmental impairments in this monogenic syndrome and establish NF1 as a quantitative trait locus for autism.
Methods
Anonymized, individual-level primary data were pooled from 6 tertiary referral centers: Washington University School of Medicine8; University of California, San Francisco9; Children’s National Health System10; University Hospital of Leuven11; The University of Manchester12; and The Children’s Hospital at Westmead. For all patients, diagnosis of NF1 was established using the National Institutes of Health diagnostic criteria.6 Data were collected for a total of 691 unique individuals. Following data cleaning and removal of reports with missing items that exceeded published reliability thresholds, 531 total patients were available for analysis. Individuals ranged in age from 2.5 to 83.9 years (median, 11 years). A total of 247 of the patients (46.5%) were males; there was no statistically significant difference in the proportion of males across sites. Detailed information on the samples collected at each site are summarized in Table 1. An ASD-enriched subsample (n = 79) recruited for inclusion in an NF1-ASD clinical trial (eTable 1 in the Supplement) was also included for the purpose of comparison factor analysis and subsequent sensitivity analysis. Data collection at each site was approved by the respective institutional review boards (University of California, Los Angeles, Institutional Review Board; University of California, San Francisco, Human Research Protection Program; Washington University Human Research Protection Office; Greater Manchester South Ethics Committee; Greater Manchester West Ethics Committee; Leuven University Hospital Ethics Committee; Children’s National Medical Center Institutional Review Board, and Sydney Children’s Hospitals Network Human Research Ethics Committee), and written informed consent was obtained for each participant. The cooperative data-sharing and analysis plan was reviewed by the Washington University Human Research Protection Office, and the analysis of pooled data was designated as nonresearch given the use of deidentified existing data.
Table 1.
INFACT Site-Specific Demographics of Patients With NF1
| Characteristic | WUSM8 | UCSF9 | CNHS10 | UHL11 | UM12 | CHW | All Sites |
|---|---|---|---|---|---|---|---|
| Recruitment population | Convenience sample from NF1 outpatient clinic | Convenience sample from UCSF genetics clinics, NF1 educational symposia at UCSF, and social media outlets | Convenience sample from patients referred to an outpatient NF1 clinic who completed intake screening package | Convenience sample recruited on a sequential basis from NF1 outpatient clinic | Population-based sample from a regional genetic registry | Convenience sample recruited on a sequential basis from a neurogenetics clinic | NA |
| ASD instrument | SRS-2 | SRS, SRS-2 | SRS, SRS-2 | SRS-2 | SRS, SRS-2 | SRS, SRS-2 | |
| ADHD instrument (pediatric) | Conners-3 | NA | VADPRS | NA | Conners-3, CPRS-R | Conners-3, CADS | |
| ADHD instrument (adult) | CAARS | NA | VADPRS | NA | NA | NA | |
| Sample size, No. | 103 | 83 | 131 | 82 | 109 | 23 | 531 |
| Age, median (range), y | 16 (3–74) | 17 (2.5–83.9) | 10 (3–53) | 9.5 (5–18) | 10 (4–16) | 10 (6–13) | 11 (2.5–83.9) |
| Sex, No. (%) | |||||||
| Male | 44 (42.7) | 32 (38.6) | 69 (52.7) | 44 (53.7) | 50 (45.9) | 8 (34.8) | 247 (46.5) |
| Female | 59 (57.3) | 51 (61.4) | 62 (47.3) | 38 (46.3) | 59 (54.1) | 15 (65.2) | 284 (53.5) |
| Age <18, No. (%), y | 56 (54.4) | 42 (50.6) | 111 (84.7) | 79 (96.3) | 109 (100) | 23 (100) | 420 (79.1) |
| Age ≥18, No. (%), y | 47 (45.6) | 41 (49.4) | 20 (15.3) | 3 (3.7) | 0 | 0 | 111 (20.9) |
| Sporadic transmission, No. (%) | 59 (57.3) | 42 (50.6)a | 41 (31.3)a | 56 (68.3) | 29 (26.6)a | 13 (56.5) | 240 (45.2)a |
| Familial transmission, No. (%) | 44 (42.7) | 29 (34.9)a | 17 (13)a | 26 (31.7) | 49 (45)a | 10 (43.5) | 175 (33)a |
| SRS T score, mean (SD) | 58.43 (12.76) | 53.81 (15.45)b | 55.33 (10.55)b | 60.76 (12.63) | 63.05 (14.35)b | 57.65 (12.29) | 58.21 (13.38) |
| SRS-2 score ≥60, No. (%) | 45 (43.7) | 23 (27.7)c | 38 (29)c | 39 (47.6) | 56 (51.4) | 7 (30.4) | 208 (39.2) |
| SRS-2 score ≥70, No. (%) | 18 (17.5) | 11 (13.3) | 13 (9.9) | 18 (22) | 32 (29.4)d | 4 (17.4) | 96 (18.1) |
| SRS-2 score ≥75, No. (%) | 13 (12.6) | 10 (12) | 8 (6.1)e | 12 (14.6) | 24 (22) | 3 (13) | 70 (13.2) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; CAARS, Conners Adult ADHD Rating Scale; CADS, Conners ADHD/DSM-IV Scales; CHW, The Children’s Hospital at Westmead; CNHS, Children’s National Health System; Conners-3, Conners, third edition; CPRS-R, Conners Parent Rating Scale–Revised; INFACT, International NF1-ASD Consortium Team; NA, not applicable; NF-1, neurofibromatosis type 1; SRS-2, Social Responsiveness Scale, second edition; UCSF, University of California, San Francisco; UHL, University Hospital of Leuven; UM, The University of Manchester; VADPRS, Vanderbilt ADHD Diagnostic Parent Rating Scale; WUSM, Washington University School of Medicine.
Total does not equal 100% due to presence of unknown values.
The University of Manchester is greater than UCSF and CNHS; P < .001.
University of California, San Francisco and CNHS are less than all other sites; P = .001.
The University of Manchester is greater than all other sites; P = .004.
CNHS is less than all other sites; P = .002.
Measurement of Quantitative Autistic Traits
The Social Responsiveness Scale, second edition (SRS-2) is an extensively validated, 65-item quantitative trait measure16 designed to ascertain the presence and severity of social communicative and repetitive behaviors that characterize ASD. Items are rated on a 4-point Likert-type scale and contribute to 2 empirically derived subscales that correspond to the criterion domains for ASD as described in the DSM-5.1 T scores of 60 or higher indicate clinically significant ASD symptoms, and T scores of 75 or higher are associated with categorical ASD diagnosis, with a sensitivity of 0.85 and specificity of 0.75 for generating a consensus diagnosis of ASD.17 For children, the ratings were completed by parents or guardians. For adults, the ratings were completed by either self-report (10 [1.8%]) or by a parent, spouse, or close friend. Ten individuals younger than 30 months and 71 with invalid SRS reports owing to missing data (median, 65 missing items; range, 7–65) were excluded from analysis.
Measurement of Attention Problems
Four versions of the Conners ADHD rating scales were used across sites: Conners, third edition,18 Conners Adult ADHD Rating Scale,19 Conners Parent Rating Scale–Revised,20 and Conners ADHD/DSM-IV Scales.21 This system exhibits robust sensitivity (92.3%) and specificity (94.5%) for generating a consensus diagnosis of ADHD.22 Items are rated on a 4-point Likert-type scale and generate categorical scores characterizing impairments in ADHD including hyperactivity, inattentiveness, and an ADHD index (AI) score, which are comparable across measurement instruments. Total AI scores of 65 or higher indicate clinically significant ADHD symptomatology.
Statistical Analysis
Categorical variables are reported as frequencies and proportions and compared using logistic regression methods. Continuously distributed traits adhering to both conventional normality assumptions and homogeneity of variances are reported as mean (SD) and compared using analysis of variance methods. Nonparametric equivalents were used for data with nonnormative distributions. Within-family association of quantitative ASD impairment was investigated using intraclass correlation, implementing a 2-way random effects model. Correlations between neurobehavioral traits were investigated using Pearson correlation coefficient. A weighted least-squares factor analysis with oblique rotation was applied to evaluate the factor structure of ASD traits with and without inclusion of the ASD-enriched sample. Highest-loading items on the first principal component generated from factor analysis of the ASD-enriched sample were used to derive a principal factor score that was subsequently tested in sensitivity analysis against a subset of cases from The University of Manchester, in which categorical research diagnoses had been previously derived.13 With the use of empirically derived SRS factors, a series of mixture models (MPlus, version 7.3)23 were used to investigate the presence of latent classes (eMethods in the Supplement).24–26 Data were analyzed in SPSS, version 23 (IBM Corp).27
Results
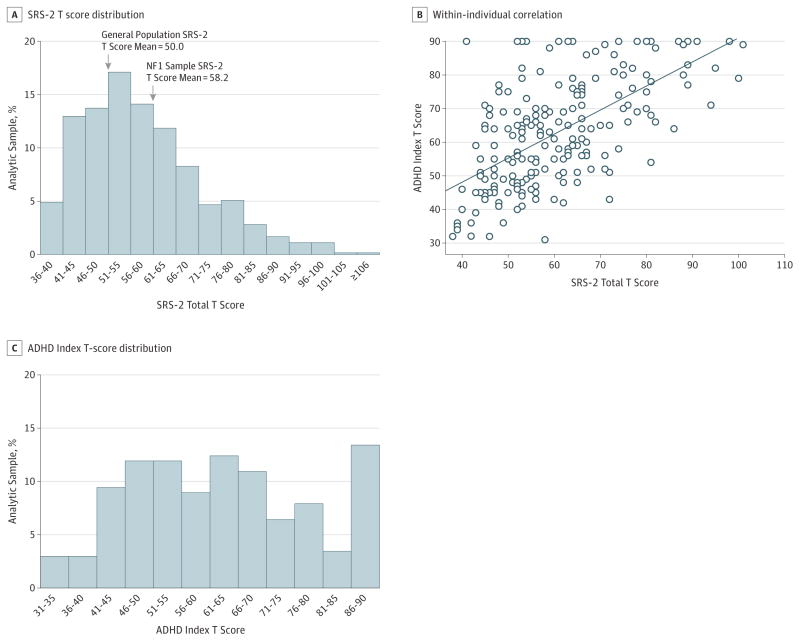
Of the 531 patients included in the analysis, 247 were male (46.5%); median age was 11 years (range, 2.5–83.9) years. The distribution of SRS-2 total scores was continuous and pathologically shifted by 0.8 SD (1.0 SD for males; 0.6 SD for females) relative to population norms (mean [SD] T score, 58.21 [13.38]) (Figure 1A). This same pathologic shift was observed when analyzing SRS-2 data across all respective levels of treatment scales and DSM-5 subscale scores (social communication and interaction and restricted interests and repetitive behaviors; eTable 2 in the Supplement). Moreover, 39.2% (95% CI, 35.1%–43.4%) of individuals with NF1 exhibited above-threshold QAT scores (T score ≥60), with 13.2% (95% CI, 10.3%–16.1%) scoring in the most severe range (T score ≥75).
Figure 1. Standardized Quantitative Trait Distribution for Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) in Neurofibromatosis Type 1 (NF1).
A, Distribution of Social Responsiveness Scale, second edition (SRS-2) total T scores for the International NF1-ASD Consortium Team (INFACT) sample (N = 531) relative to the general population. B, Within-individual correlation between SRS-2 total T scores and ADHD Index T scores; r = 0.61. The solid line represents the line of best fit. C, Distribution of ADHD Index T scores for the INFACT sample (n = 207). Distributions for the limited sample (including only individuals with both ASD and ADHD trait scores) are available in eFigure 1 in the Supplement.
Males had statistically significantly higher mean SRS-2 total scores (mean difference, 3.59; 95%CI, 1.31–5.86) and statistically significantly higher scores for all quantitative subscales relative to females (P = .002), such as the Social Cognition T Score (mean difference, 2.87; 95% CI, 0.54–5.20), Social Motivation T Score (mean difference, 2.36; 95% CI, 0.30–4.42), Social Communication T Score (mean difference, 4.14; 95% CI, 1.89–6.39), Social Awareness T Score (mean difference, 2.67; 95% CI, 0.55–4.79), and Restricted Interests and Repetitive Behaviors T Score (mean difference, 3.07; 95%CI, 0.75–5.39). However, when categorically evaluating symptomatology within the most severe range (T score ≥75), the male to female sex ratio was only 1.6:1 and approached 1.25:1 when a broader autism phenotype (T score ≥60) was considered, which is significantly narrower than the epidemiologically determined ASD sex ratio of 4:1.2 When we adjusted for potential site-specific effects, the attenuated sex ratio persisted (adjusted odds ratio, 0.55; 95% CI, 0.33–0.92).
In contrast to reductions in SRS-2 scores that typically occur during the interval from preschool to school years, QAT burden peaked in children with NF1 between the ages of 8 and 17 years (T score ≥70) (68 of 274 [24.8%]), with mean total scores that were 6.20 points (95% CI, 3.50–8.90) higher than in children aged 2.5 to 7 years old and 7.09 points (95%CI, 4.20–10.02) higher than in adults. The AI scores similarly trended higher among children aged 8 to 17 years relative to those aged 2.5 to 7 years, although this difference did not reach statistical significance (P = .06). Adults had consistently lower inattentive and hyperactivity scores than children (median inattentive T score, 54 [range, 28–90] vs 65 [range, 40–90]; hyperactivity T score, 46 [range, 32–71] vs 61 [range, 40–90]; both P = .001), similar to general population samples.28
Quantitative ASD and ADHD trait scores were moderately correlated within individual participants (r = 0.61) (Figure 1B) and were proportionally elevated in those with above-threshold QAT scores (eTable 3 in the Supplement). However, despite this correlation, quantitative ASD and ADHD trait scores generated highly contrasting distributions (Figure 1C). Of 207 patients with available ADHD data (eFigure 1 in the Supplement), 94 (45.4%; 95% CI, 38.8%–52.4%) exhibited above-threshold ADHD scores, and, in categorical evaluation of severe ADHD symptoms (AIT score ≥65), the male to female ratio was 1:1. As expected, individuals with above-threshold ADHD scores demonstrated statistically significantly higher QAT scores (mean difference, 13.70; 95% CI, 10.34–17.06) compared with those with lower ADHD trait scores; however, more than half of the patients with available ADHD data (111 [53.6%]) exhibited a QAT burden in the normal range (T score <60). In the absence of clinically relevant ADHD symptoms, SRS-2 scores remained pathologically shifted by 0.4 SD for both sexes relative to the general population.
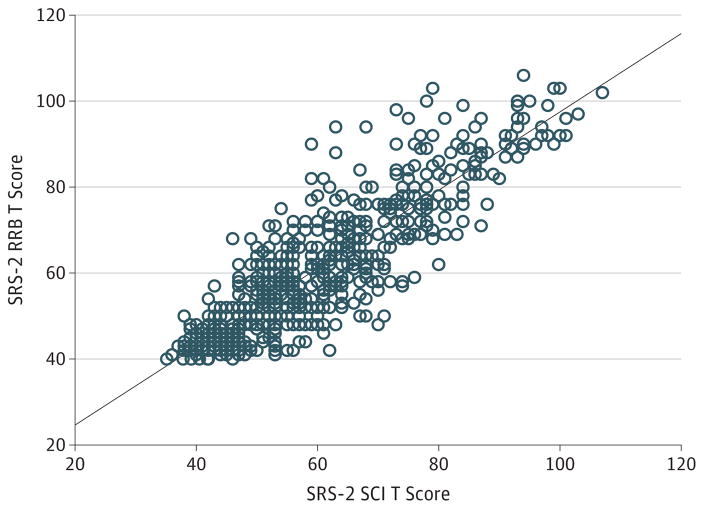
To investigate the symptom structure of ASD in NF1, a series of factor analyses were conducted. Factor analysis of data from patients with NF1 unselected for ASD extracted 13 principal components. The first principal component explained 30.9%of the variance in SRS-2 scores (eTable 4 in the Supplement), and the highest-loading items (loadings >0.5) encompassed both ASD core domains (Table 2). This factor structure represents a typical pattern for a continuously distributed trait with a unitary factor structure,29 an interpretation further supported by the strong correlation observed between DSM-5 subdomains in the NF1 sample (r = 0.84) (Figure 2), although fewer of the pathognomonic symptoms of ASD were represented in this NF1 item set than in previously published24,29 factor analyses involving clinical family samples of idiopathic ASD. Factor analysis including the ASD-enriched subsample (610 individuals) revealed comparatively stronger results with extraction of 11 principal components (eTable 5A in the Supplement), the first of which explained 34.3% of the variance in SRS-2 scores. Highest-loading items again represented the full ASD phenotype (eTable 5B in the Supplement).
Table 2.
Exploratory Factor Analysis Item Loadings on Principal Components 1–6a
| SRS Item | Domain | Component | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Factor 1 | |||||||
| Literal, does not understand the real meaning | SCI | 0.72 | 0.01 | 0.02 | 0.08 | −0.04 | 0.02 |
| Does not know when others take advantage | SCI | 0.70 | −0.06 | 0.03 | 0.17 | 0.04 | 0.05 |
| Is not physically well coordinated | RRB | 0.61 | −0.13 | −0.18 | 0.08 | 0.14 | 0.61 |
| Clings to adults, seems too dependent | SCI | 0.58 | −0.07 | −0.24 | −0.11 | 0.11 | 0.58 |
| Wanders aimlessly, 1 activity to another | SCI | 0.56 | −0.11 | 0.14 | −0.07 | −0.15 | 0.56 |
| Does extremely well at a few tasks but not most | RRB | 0.56 | −0.003 | −0.11 | 0.05 | 0.10 | 0.56 |
| Concentrates on parts, does not see the whole | SCI | 0.53 | 0.12 | 0.12 | 0.07 | 0.11 | 0.53 |
| Factor 2 | |||||||
| Recognizes when something is unfair | SCI | 0.07 | 0.65 | 0.01 | −0.12 | −0.01 | 0.13 |
| Responds appropriately to others’ mood changes | SCI | −0.06 | 0.64 | −0.09 | 0.03 | 0.13 | −0.04 |
| Is able to imitate others’ actions | SCI | −0.04 | 0.62 | 0.07 | −0.004 | 0.07 | 0.05 |
| Offers comfort when others are sad | SCI | −0.18 | 0.58 | 0.20 | −0.09 | 0.02 | 0.04 |
| Understands tone of voice, facial expression | SCI | −0.14 | 0.57 | −0.01 | 0.16 | 0.04 | −0.004 |
| Has a sense of humor, understands jokes | SCI | 0.11 | 0.51 | 0.05 | −0.13 | −0.14 | 0.09 |
| Factor 3 | |||||||
| Walks between 2 people who are talking | SCI | −0.01 | 0.08 | 0.81 | 0.02 | −0.06 | 0.07 |
| Difficulty with changes in routine | RRB | −0.06 | −0.06 | 0.76 | 0.06 | 0.04 | 0.10 |
| Uses unusual tone: like robot or lecture | SCI | −0.22 | −0.04 | 0.74 | 0.06 | 0.04 | −0.13 |
| Has good self-confidence | SCI | 0.12 | −0.08 | 0.51 | −0.05 | 0.01 | −0.09 |
| Factor 4 | |||||||
| Avoids people who want to be emotionally close | SCI | 0.02 | −0.04 | 0.03 | 0.76 | 0.11 | 0.03 |
| Wanders aimlessly, 1 activity to another | SCI | 0.17 | −0.03 | 0.10 | 0.72 | −0.13 | 0.08 |
| Concentrates on parts, does not see the whole | SCI | 0.02 | −0.01 | −0.05 | 0.66 | −0.08 | 0.05 |
| Unusual sensory interests (mouthing/spinning) | RRB | 0.11 | −0.10 | 0.01 | 0.50 | 0.01 | 0.07 |
| Factor 5 | |||||||
| Is overly suspicious | SCI | 0.26 | 0.06 | −0.21 | −0.05 | 0.62 | −0.01 |
| Stares or gazes off into space | SCI | −0.05 | 0.03 | 0.15 | −0.05 | 0.55 | 0.02 |
| Is too silly or laughs inappropriately | SCI | 0.18 | 0.02 | 0.06 | −0.01 | 0.54 | 0.10 |
| Factor 6 | |||||||
| Talks around the subject | SCI | 0.09 | 0.05 | −0.02 | 0.13 | 0.02 | 0.85 |
| Avoids starting social interactions | SCI | 0.02 | 0.15 | −0.01 | 0.04 | −0.05 | 0.66 |
Abbreviations: RRB, restricted interests and repetitive behaviors; SCI, social communication and interaction; SRS, Social Responsiveness Scale.
Excluding autism spectrum disorder–enriched sample.
Figure 2. Within-Individual Correlation of DSM-5 Subdomains.
Correlation between social communication and interaction (SCI) T scores and restricted interests and repetitive behaviors (RRB) T scores as measured by Social Responsiveness Scale, second edition (SRS-2); r = 0.84. The solid line represents the line of best fit.
To define the association between the QAT burden and autism per se, we tested the principal factor score derived from these highest-loading items in a sensitivity analysis against a subset of cases (n = 47) in which categorical research diagnoses had been derived using Autism Diagnostic Interview–Revised and Autism Diagnostic Observation Schedule assessments.13 The principal factor score demonstrated no improvement in sensitivity or specificity for categorical ASD diagnosis when we substituted the factor score for the total score derived from all SRS-2 items (area under the curve,0.72 vs 0.76) (eFigure 2 in the Supplement).13
To investigate the presence of latent classes within the INFACT sample, a mixture model was implemented that used fit statistics for 1 to 6 class solutions based on empirically derived SRS factors (eTable 6 in the Supplement). Fit improved up to the 5-class solution for most information criteria; however, the largest improvement was from 1 to 2 classes, and classification was largest for the 2- and 3-class models. The 3-class model, while showing slightly better fit and the best classification entropy, had 1 small class representing less than 7%of the sample, so the more conservative 2-class solution was favored.
The 2-class solution yielded 1 dominant class (class 1: 81.1%) and 1 smaller class (class 2: 18.9%) that had statistically significantly higher mean scores for each SRS-2 factor (P < .001) (eFigure 3 in the Supplement) and statistically significantly elevated mean SRS-2 T scores (mean difference, 19.97; 95%CI, 17.16–22.79) compared with the larger class. Individuals in class 2 were older than those in class 1 (mean difference, 7.17 years; 95%CI, 3.70–10.64), and, as expected, there were proportionally more males in the group with elevated SRS-2 scores (63 [61.8%] vs 186 [42.4%]; P < .001). When hierarchical regression analysis was used, SRS-2 scores independently contributed 34.9% of the variance in empirical classifications, and ADHD symptom scores independently contributed 2.5%of the variance in empirical classifications (P = .002).
Data on IQ (derived from Wechsler Intelligence Scales30,31) were available in subsets of individuals from 5 of the 6 participating sites. Findings were concordant with previously reported IQ range and prevalence of intellectual disability within the NF1 population,14 with mean full-scale IQ in 81 patients of 85.8 (range, 56–117); 9 of these patients (11.1%) had full-scale IQ lower than 70. Mean verbal IQ in 112 individuals was 89.9 (range, 55–126). No statistically significant correlation was observed between full-scale IQ or mean verbal IQ and QAT scores in this NF1 cohort (eFigure 4 in the Supplement).
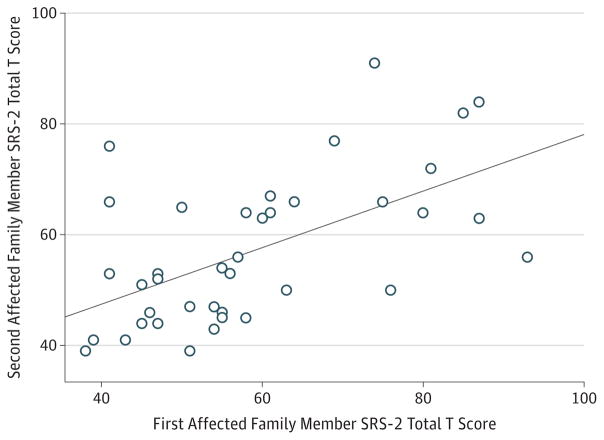
Finally, we analyzed SRS-2 data from 39 available pairings of first-degree relatives with NF1 representing various ages, sex, and familial relationships (eTable 7 in the Supplement) and observed an infraclass correlation coefficient of 0.73 (Figure 3). This association represents a 3-foldincrease in the proportion of variance explained by a first-degree relative score compared with previously published results from general population studies of dizygotic twins32 and from clinical family studies involving nontwin siblings and proband/parent pairs.33,34
Figure 3. Within-Family Association of Quantitative Autistic Trait Scores.
Correlation between Social Responsiveness Scale, second edition (SRS-2) total T scores for first-degree relative pairings concordantly affected by neurofibromatosis type 1 (n = 39 pairs); intraclass correlation coefficient, 0.73. The solid line represents the line of best fit.
Discussion
To our knowledge, the INFACT sample constitutes the largest NF1 patient data set in which quantitative ASD burden has been systematically investigated and consequently enables a number of important conclusions not possible using smaller data sets.
First, we demonstrated that QAT scores are continuously distributed and pathologically shifted in NF1 compared with the general population and that the proportion of patients with NF1 with QAT scores at or above the first percentile (T score ≥75) is more than 13 times that observed in the general population. These findings have important implications for the clinical care of patients with NF1, revealing that the NF1 gene likely functions as a quantitative trait locus for ASD and that NF1 is unequivocally associated with highly elevated ASD symptom burden.
A larger class of individuals with highly elevated QAT scores (18.9%) was derived from mixture modeling of the SRS-2 data, mapping to the proportion of individuals within the INFACT sample with SRS-2 T scores of 70 or above (18.1%; greater than the second percentile for the general population distribution). This finding suggests an empirically derived phenotypic threshold for ASD in NF1 and, when applied to the school-aged cohort, produces a similar proportion of above-threshold affectation (24.8%) to previously reported classification prevalences obtained using standard diagnostic instruments (24.9%).13
Second, we observed that QAT scores were continuously distributed and pathologically shifted for both sexes. At the pathologic extreme, we observed a 1.6:1 male to female sex ratio, which is attenuated relative to that observed in idiopathic ASD. Taken together, these findings suggest that the hypothesized female protective effect 35 may be muted in NF1 or that NF1 mutations are deleterious enough to overcome sex specific factors that reduce phenotypic expression of many forms of inherited liability in females. This finding is particularly interesting in light of other sexually dimorphic traits reported in individuals with NF1.36
Third, as previously reported,8,9,12,14 we identified significant quantitative ADHD burden in the INFACT sample and observed that ADHD tends to track with autistic traits in the context of NF1. However, despite the substantial correlation observed, these symptom clusters exhibited contrasting trait distributions, differing sex ratios, and highly discrepant contributions to empirical classifications, and we observed that most individuals with above-threshold ADHD symptoms scored within the normal range for QATs. Collectively, these findings suggest that the co-occurrence of ASD and ADHD in NF1 is most appropriately viewed as a function of the mutation causing both types of symptoms (much as it causes diverse oncologic and orthopedic features) rather than as a manifestation of measurement confound.37
Fourth, we observed that autistic symptoms in NF1 conform to the same unitary factor structure observed for idiopathic ASD—reinforced by the correlation observed between social communication and interaction and repetitive behavior domain scores in NF1—although the highest-loading items on the first principal component appeared somewhat less autism specific than those observed in idiopathic ASD from the Simons Simplex Collection (eTables 1, 2, 5C, and 5D in the Supplement), suggestive of a unique NF1-ASD phenotype. These results represent a critical case in principle that a single quantitative trait locus is capable of producing the full complement of ASD symptoms across the entire range of severity with which they occur in nature and extends convergent results obtained from in-person ASD phenotyping.13 Although the lack of confirmatory clinical diagnoses at most participating sites is a relative limitation of this study, the systematic use of a highly validated quantitative screening tool in a large international sample afforded an unprecedented opportunity to investigate the quantitative autistic phenotype of NF1 that is not otherwise possible with standard diagnostic instruments.
Additional limitations of this study include the lack of available data on NF1 severity and lack of general psychopathology data. However, earlier reports8,13 on subsets of this cohort demonstrated no association between QAT burden and NF1 severity, and we recapitulate the independence of QAT ratings from measurement of IQ as similarly seen in idiopathic ASD.38–40 Although deficits in reciprocal social behavior have been demonstrated41,42 to be genetically independent from non-ADHD domains of psychopathology, significant elevations have been observed in patients with anxiety disorder. To this end, the lack of general psychopathology data in this cohort limits a comprehensive characterization of the NF1-ASD phenotype and should be a focus of future research.
Finally, the INFACT data set replicated a prior observation8 of a striking association in QAT burden between first-degree relatives with NF1 (intraclass correlation coefficient, 0.73). This association is substantially higher than previously observed32,43 on the basis of family genetic background and suggests a high degree of mutational specificity for ASD severity. A remarkable feature of this association is that it operates across the entire SRS distribution (from unaffected to severe) rather than just within a constrained range of clinical affectation for ASD. This intra familial specificity is supported by studies demonstrating emerging NF1 genotype-phenotype correlations in both humans and mice,44,45 and elucidating the mechanisms underlying these gene mutation–specific contributions may yield improved risk assessment and management strategies for an otherwise clinically unpredictable condition.
Conclusions
These data provide confirmation that the diversity of mutations that give rise to NF1 function as quantitative trait loci for ASD and, to our knowledge, are only the second demonstration using an adequately powered research sample (ie, Fragile X Mental Retardation [FMR1]) that a mutation in a single gene results in a neurodevelopmental syndrome of variable severity encompassing the complete ASD phenotype. Clinicians should be alerted to the high frequency of this disabling comorbidity of NF1, and the scientific community should recognize the potential for this monogenic disorder to provide new insights into the pathobiology of idiopathic autism.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NIH) under Award U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University (Dr Constantino), Schnuck Markets, Inc, the Neurological Sciences Academic Development Award at Washington University School of Medicine under Award K12 NS001690 (Dr Morris), the NIH New Innovator under Award 1DP2OD007449 (Dr Weiss), the Opening the Future grant of Katholieke Universiteit Leuven (Dr Legius), the Manchester Biomedical Research Center Clinical Research Fellowship (Drs Garg and Green), and the Central Manchester Foundation Trust Research and Innovation Award (Drs Green and Huson).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Morris had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Acosta, Green, Legius, Payne, Plasschaert, Weiss, Gutmann, Constantino.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Morris, Green, Frazier, Gutmann, Constantino.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Morris, Frazier, Zhang, Constantino.
Administrative, technical, or material support: Acosta, Garg, Huson, Legius, North, Plasschaert, Gutmann, Constantino.
Study supervision: Acosta, Garg, Green, Legius, Payne, Plasschaert, Gutmann, Constantino.
Conflict of Interest Disclosures: Dr Frazier reported receiving federal funding or research support from, acting as a consultant to, receiving travel support from, and/or receiving a speaker’s honorarium from the Cole Family Research Fund, Simons Foundation, Ingalls Foundation, Forest Laboratories, Ecoeos, IntegraGen, Kugona LLC, Shire Development, Bristol-Myers Squibb, National Institutes of Health, and the Brain and Behavior Research Foundation. Dr Constantino reported receiving royalties from Western Psychological Services for commercial sales and distribution of the Social Responsiveness Scale–2. No other conflicts were reported.
Additional Information: The authors are all members of the International NF1-ASD Consortium Team (INFACT).
Additional Contributions: We also acknowledge the Children’s Tumor Foundation for facilitating the INFACT meetings.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Christensen DL, Baio J, Van Naarden Braun K, et al. Centers for Disease Control and Prevention (CDC) Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisula E, Danielewicz D, Kawa R, Pisula W. Autism spectrum quotient, coping with stress and quality of life in a non-clinical sample—an exploratory report. Health Qual Life Outcomes. 2015;13:173. doi: 10.1186/s12955-015-0370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SH, Ripke S, Neale BM, et al. Cross-Disorder Group of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311(17):1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institutes of Health Consensus Development Conference statement: neurofibromatosis. Arch Neurol. 1988;45(5):575–578. [PubMed] [Google Scholar]
- 7.Uusitalo E, Hammais A, Palonen E, et al. Neurofibromatosis type 1 gene mutation analysis using sequence capture and high-throughput sequencing. Acta Derm Venereol. 2014;94(6):663–666. doi: 10.2340/00015555-1843. [DOI] [PubMed] [Google Scholar]
- 8.Constantino JN, Zhang Y, Holzhauer K, et al. Distribution and within-family specificity of quantitative autistic traits in patients with neurofibromatosis type 1. J Pediatr. 2015;167(3):621–626. doi: 10.1016/j.jpeds.2015.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adviento B, Corbin IL, Widjaja F, et al. Autism traits in the RASopathies. J Med Genet. 2014;51(1):10–20. doi: 10.1136/jmedgenet-2013-101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh KS, Vélez JI, Kardel PG, et al. Symptomatology of autism spectrum disorder in a population with neurofibromatosis type 1. Dev Med Child Neurol. 2013;55(2):131–138. doi: 10.1111/dmcn.12038. [DOI] [PubMed] [Google Scholar]
- 11.Plasschaert E, Descheemaeker MJ, Van Eylen L, Noens I, Steyaert J, Legius E. Prevalence of autism spectrum disorder symptoms in children with neurofibromatosis type 1. Am J Med Genet B Neuropsychiatr Genet. 2015;168(1):72–80. doi: 10.1002/ajmg.b.32280. [DOI] [PubMed] [Google Scholar]
- 12.Garg S, Lehtonen A, Huson SM, et al. Autism and other psychiatric comorbidity in neurofibromatosis type 1: evidence from a population-based study. Dev Med Child Neurol. 2013;55(2):139–145. doi: 10.1111/dmcn.12043. [DOI] [PubMed] [Google Scholar]
- 13.Garg S, Green J, Leadbitter K, et al. Neurofibromatosis type 1 and autism spectrum disorder. Pediatrics. 2013;132(6):e1642–e1648. doi: 10.1542/peds.2013-1868. [DOI] [PubMed] [Google Scholar]
- 14.Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65(7):1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- 15.Payne JM, Hyman SL, Shores EA, North KN. Assessment of executive function and attention in children with neurofibromatosis type 1: relationships between cognitive measures and real-world behavior. Child Neuropsychol. 2011;17(4):313–329. doi: 10.1080/09297049.2010.542746. [DOI] [PubMed] [Google Scholar]
- 16.Constantino JN, Gruber CP. Social Responsiveness Scale. 2. Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- 17.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview–Revised. J Autism Dev Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 18.Conners CK. Conners. 3. Toronto, ON: Multi-Health Systems; 2008. [Google Scholar]
- 19.Conners CK, Ehrhardt D, Sparrow E. CAARS Adult ADHD Rating Scales. Toronto, ON: Multi-Health Systems; 1999. [Google Scholar]
- 20.Conners CK. Conners Parent Rating Scale—Revised. Toronto, ON: Multi-Health Systems; 1997. [Google Scholar]
- 21.Conners CK. Conners ADHD/DSM-IV Scales. North Tanawanda, NY: Multi-Health Systems; 1997. [Google Scholar]
- 22.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 23.Muthen LK, Muthen BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthen & Muthen; 1998–2012. [Google Scholar]
- 24.Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, Constantino JN. Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the Social Responsiveness Scale–2. Autism. 2014;18(1):31–44. doi: 10.1177/1362361313500382. [DOI] [PubMed] [Google Scholar]
- 25.Nylund KL, Asparouhoy T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling. 2007;14(4):535–569. [Google Scholar]
- 26.Yang C. Evaluating latent class analysis models in qualitative phenotype identification. Comput Stat Data Anal. 2006;50:1090–1104. [Google Scholar]
- 27.IBM SPSS Statistics (for Windows) [computer program]. Version 23.0. Armonk, NY: IBM Corp; 2015. [Google Scholar]
- 28.Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. 2010;49(3):217–228. [PMC free article] [PubMed] [Google Scholar]
- 29.Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. J Child Psychol Psychiatry. 2004;45(4):719–726. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. The Wechsler Intelligence Scale for Children. 3. San Antonio, TX: Psychological Corp; 1991. [Google Scholar]
- 31.Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: Psychological Corp, Harcourt Brace & Co; 1999. [Google Scholar]
- 32.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 33.Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57(6):655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Frazier TW, Thompson L, Youngstrom EA, et al. A twin study of heritable and shared environmental contributions to autism. J Autism Dev Disord. 2014;44(8):2013–2025. doi: 10.1007/s10803-014-2081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacquemont S, Coe BP, Hersch M, et al. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am J Hum Genet. 2014;94(3):415–425. doi: 10.1016/j.ajhg.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diggs-Andrews KA, Brown JA, Gianino SM, Rubin JB, Wozniak DF, Gutmann DH. Sex is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann Neurol. 2014;75(2):309–316. doi: 10.1002/ana.24093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Constantino JN, Frazier TW. The observed association between autistic severity measured by the Social Responsiveness Scale (SRS) and general psychopathology—a response to Hus et al (2013) J Child Psychol Psychiatry. 2013;54(6):695–697. doi: 10.1111/jcpp.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamio Y, Inada N, Moriwaki A, et al. Quantitative autistic traits ascertained in a national survey of 22 529 Japanese schoolchildren. Acta Psychiatr Scand. 2013;128(1):45–53. doi: 10.1111/acps.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Constantino JN, Lavesser PD, Zhang Y, Abbacchi AM, Gray T, Todd RD. Rapid quantitative assessment of autistic social impairment by classroom teachers. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1668–1676. doi: 10.1097/chi.0b013e318157cb23. [DOI] [PubMed] [Google Scholar]
- 40.Garg S, Plasschaert E, Descheemaeker MJ, et al. Autism spectrum disorder profile in neurofibromatosis type I. J Autism Dev Disord. 2015;45(6):1649–1657. doi: 10.1007/s10803-014-2321-5. [DOI] [PubMed] [Google Scholar]
- 41.Constantino JN, Hudziak JJ, Todd RD. Deficits in reciprocal social behavior in male twins: evidence for a genetically independent domain of psychopathology. J Am Acad Child Adolesc Psychiatry. 2003;42(4):458–467. doi: 10.1097/01.CHI.0000046811.95464.21. [DOI] [PubMed] [Google Scholar]
- 42.Duvekot J, van der Ende J, Constantino JN, Verhulst FC, Greaves-Lord K. Symptoms of autism spectrum disorder and anxiety: shared familial transmission and cross-assortative mating. J Child Psychol Psychiatry. 2016;57(6):759–769. doi: 10.1111/jcpp.12508. [DOI] [PubMed] [Google Scholar]
- 43.Hoekstra RA, Bartels M, Verweij CJ, Boomsma DI. Heritability of autistic traits in the general population. Arch Pediatr Adolesc Med. 2007;161(4):372–377. doi: 10.1001/archpedi.161.4.372. [DOI] [PubMed] [Google Scholar]
- 44.Rojnueangnit K, Xie J, Gomes A, et al. High incidence of Noonan syndrome features including short stature and pulmonic stenosis in patients carrying NF1 missense mutations affecting p.Arg1809: genotype-phenotype correlation. Hum Mutat. 2015;36(11):1052–1063. doi: 10.1002/humu.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toonen JA, Anastasaki C, Smithson LJ, et al. NF1 germline mutation differentially dictates optic glioma formation and growth in neurofibromatosis-1. Hum Mol Genet. 2016;25(9):1703–1713. doi: 10.1093/hmg/ddw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.