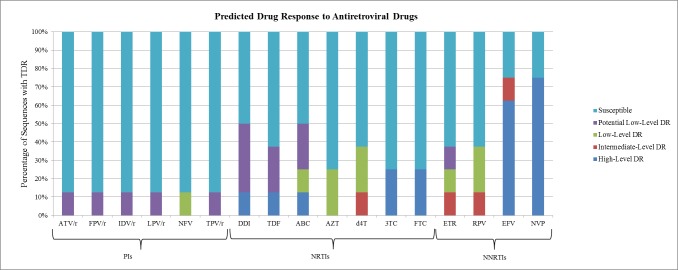
Fig 1. Predicted antiretroviral (ARV) drug responses of eight persons with TDR mutations.
Drug responses were based on the Stanford HIVdb v7.0 report. The y-axis indicates the percentage number of sequences with TDR while the x-axis indicates the different ARV drug classes that were affected by the TDR mutations. Drugs that were unaffected by any particular TDR were excluded from the analyses. TDR—Transmitted Drug Resistance; PIs—Protease Inhibitors; NRTIs—Nucleoside Reverse Transcriptase Inhibitors; NNRTIs—Non-Nucleoside Reverse Transcriptase Inhibitors; ATV/r—Boosted Atazanavir; FPV/r—Boosted Fosamprenavir; IDV/r—Boosted Indinavir; LPV/r—Boosted Lopinavir; NFV—Nelfinavir; TPV/r—Boosted Tipranavir; r—Ritonavir; 3TC—Lamivudine; ABC—Abacavir; AZT—Zidovudine; D4T—Stavudine; DDI—Didanosine; FTC—Emtricitabine; TDF—Tenofovir; EFV—Efavirenz; ETR—Etravirine; NVP—Nevirapine; RPV—Rilpivirine.