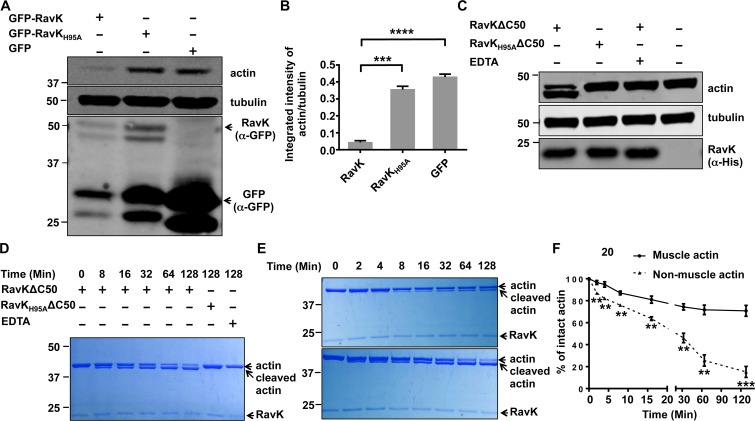
Fig 4. The cleavage of actin by RavK.
A. Expression of RavK reduced the level of actin in COS-1 cells. Indicated proteins were expressed in COS-1 cells for 24 hours and cleared cell lysates were subjected to SDS/PAGE and immunoblotting with GFP-specific and actin-specific antibodies, respectively. α-tubulin was probed as a loading control. B. The intensity of the bands corresponding to actin and tubulin was measured with ImageJ and the intensity ratio between actin and tubulin revealed the relative actin level in cells of the relevant samples. All results are from three independent experiments. Error bars represent standard error of the mean (SEM). ***, p<0.001, ****, p<0.0001. C. Recombinant RavKΔC50 cleaved actin in lysates of COS-1 cells. Indicated proteins were added to COS-1 cell lysates and the reactions were allowed to proceed for 1 h at 22°C, EDTA was added to the indicated samples. Samples were separated by SDS/PAGE and detected by immunoblotting with an actin-specific antibody. α-tubulin was probed as a loading control. D. Recombinant RavKΔC50 cleaved actin in vitro in an HEXXH motif-dependent manner. 10-μg human non-muscle actin was incubated with 1-μg RavKΔC50 or RavKH95AΔC50 for the indicated time and the mixtures separated by SDS/PAGE were stained with Coomassie brilliant blue. EDTA was added into the samples at the beginning when indicated. E. The cleavage of human non-muscle actin and rabbit muscle actin by RavKΔC50. The in vitro cleavage was performed similarly as described in D. Upper panel: Muscle actin; Lower panel: Non-muscle actin. F. RavKΔC50 cleaves human non-muscle actin more efficiently than rabbit muscle actin. The percentage of intact actin/total actin was calculated with ImageJ. All results were from three independent experiments. *, p<0.05, ***, p<0.001.