Abstract
We reported a cysteine-based ligation strategy to generate monoubiquitinated protein while preserving the native cysteines on the acceptor protein. In mono-ubiquitination of proliferating cell nuclear antigen (PCNA) this method negates the requirement of mutating the native cysteines on PCNA. The chemically ubiquitinated PCNA contains a non-cleavable linkage of the same length as the native isopeptide linkage. It also retains normal function as the native Ub-PCNA in stimulating the ATPase activity of replication factor C (RFC) and lesion bypass synthesis by Polη. This method may be adapted for chemical ubiquitination of other proteins and for site-specific modification of a target protein at a specific site through sulfhydryl chemistry.
Keywords: ubiquitination, chemical ligation, caged cysteine, photodeprotection, translesion DNA synthesis
Graphical Abstract
We demonstrate a novel cysteine-based ligation strategy to generate monoubiquitinated proliferating cell nuclear antigen (PCNA) with a non-cleavable linkage while preserving the native cysteines on PCNA.
Ubiquitination regulates many essential biological pathways including protein degradation, localization and DNA repair1. To study the role of protein ubiquitination, sufficient amount of homogeneous ubiquitinated protein is required. Different methods were developed for generating stand-alone ubiquitin chains as previously reviewed2,3. Enzymatic and chemical methods have also been reported to produce monoubiquitinated4–26 and polyubiquitinated27–30 substrate proteins. Among them, disulfide bond mediated ubiquitin ligation were successfully used to produce monoubiquitinated H2B9, PCNA10, α-synuclein16, and Ras20, as well as polyubiquitinated PCNA29 and α-Globin28. In this strategy, the chemical ligation of Ub required the introduction of a unique cysteine to replace the native lysine and simultaneous mutations of the surface native cysteines to serines in the acceptor protein. Although sufficient amount of homogenous ubiquitinated protein has been generated for both activity assays and biophysical studies, this method has two limitations. First, the disulfide bond linkage between ubiquitin and the acceptor protein is not stable at high concentrations of reducing agent. Second, to achieve specific ubiquitination, the surface cysteines in the acceptor proteins need to be mutated to serines. This is feasible for some but not all proteins without affecting the folding, structure and activity of the acceptor proteins.
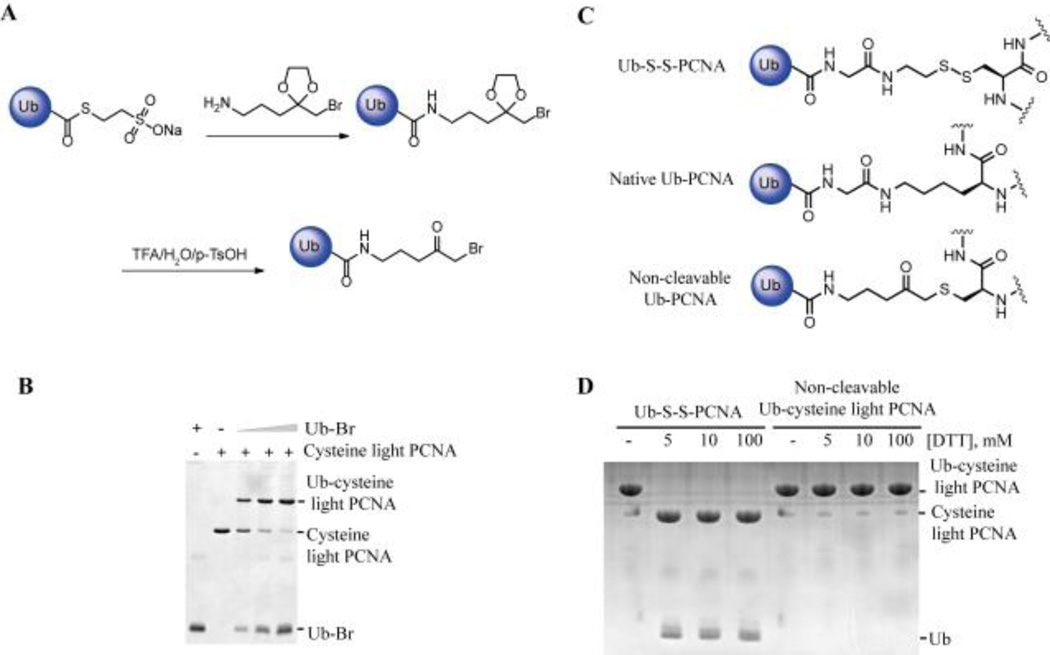
To achieve the linkage stability of ubiquitinated protein to reducing agent, ubiquitin with a non-cleavable linker (Ub-Br) (Fig. 1A) was developed following a detailed protocol described in the supplementary information. We first tested the ligation efficiency of Ub-Br with the previously generated cysteine light yeast PCNA (C22S/C30S/C62S/C81S-K164C)10 in which all the cysteines were mutated to serines and the lysine 164 was replaced with cysteine. Ub-Br was ligated to Cys164 on cysteine light PCNA efficiently (Fig. 1B). The length of the linker equals that of the native linkage (Fig. 1C). Importantly, the chemically ubiquitinated cysteine light PCNA with the non-cleavable linker is resistant to reducing agent and there was no cleavage even under 100 mM DTT treatment (Fig. 1D). In comparison, the disulfide linked Ub-PCNA was completely cleaved after incubation with 5 mM DTT for 20 minutes (Fig. 1D).
Figure 1.
Monoubiquitinated cysteine light PCNA with non-cleavable linker. (A) Generation of Ub-Br with a non-cleavable linker. (B) Ligation of Ub-Br with cysteine light PCNA (C22S/C30S/C62S/C81S-K164C). Cysteine light PCNA was incubated with increasing concentrations of Ub-Br at room temperature for 2 hours. The products were resolved on a 20% SDS-PAGE gel and stained with Coomassie blue. (C) Comparison of the linkage in the native Ub-PCNA, disulfide-linked Ub-S-S-PCNA and non-cleavable Ub-PCNA. (D) Stability of Ub-cysteine light PCNA with the non-cleavable linker treated with DTT. Ub-S-S-PCNA or non-cleavable Ub-PCNA (cysteine light) was incubated with increasing concentrations of DTT at room temperature for 20 minutes. Proteins were resolved on a 20% SDS-PAGE gel and stained with Coomassie blue.
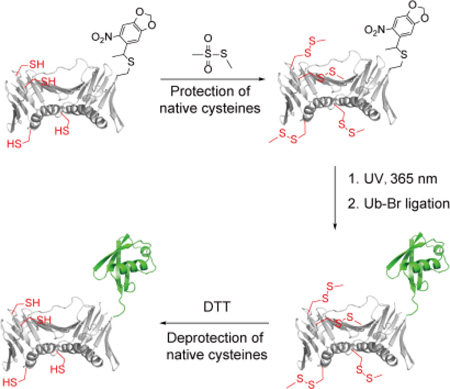
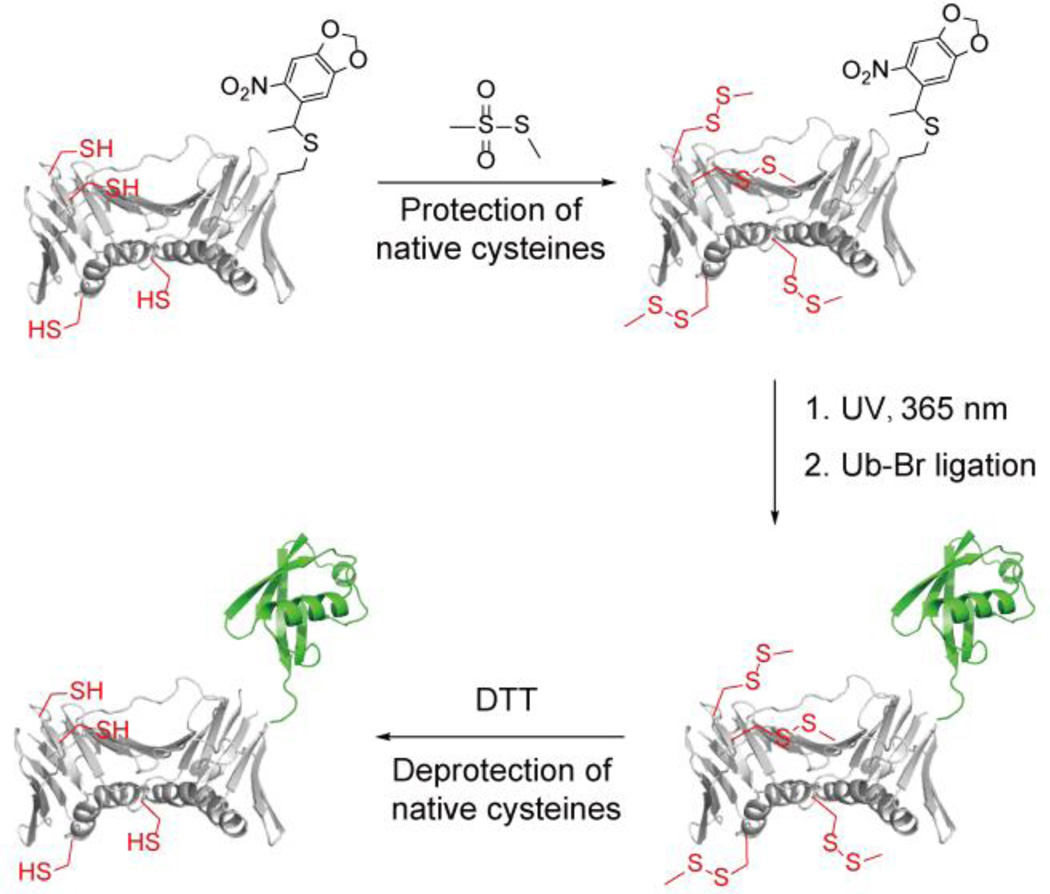
To avoid mutating the native cysteines to serines in PCNA, we developed a new chemical approach to generate monoubiquitinated PCNA as illustrated in Fig. 2. In this method a caged cysteine is genetically incorporated into PCNA to replace the Lys 164 following a reported procedure31. To achieve site-specific ubiquitination, instead of site-directed mutation to serines, all native cysteines are protected by methyl methanethiosulfonate (MMTS). Next the caging group on Cys 164 can be removed by UV irradiation, which allows the subsequent ligation with Ub-Br. Following the ubiquitin conjugation, the methylsulfanyl groups can be removed by treatment with DTT to regenerate the native cysteines.
Figure 2.
Proposed chemical method to generate monoubiquitinated PCNA. For clarity only one of the three subunits of the PCNA homotrimer is shown here.
To introduce the caged cysteine to PCNA at position 164 a genetic suppression system was used to incorporate the unnatural amino acid S-[(R,S)-1-{4',5'-(methylenedioxy)-2'-nitrophenylethyl]-L-cysteine. psfHis6-PCNA164TAGPylT and pBK-PCC2RS were co-transformed into TOP10 cells. The psfHis6-PCNA164TAGPylT plasmid contains the tRNACUA and His6-PCNA with an amber TAG codon introduced at position 164. pBK-PCC2RS encodes the synthetase PCC2RS for incorporation of S-[(R,S)-1-{4',5'-(methylenedioxy)-2'-nitrophenyl}ethyl]-L-cysteine31. The cells were cultured in the presence of 2 mM S-[(R,S)-1-{4',5'-(methylenedioxy)-2'-nitrophenyl}ethyl]-L-cysteine, which was synthesized following a reported protocol31. Protein expression was induced by L-arabinose and caged Cys-PCNA was purified by cobalt affinity resin with a yeild of approximate 3 mg protein from 1 L cell clulture. The purity of caged Cys-PCNA was analyzed on a SDS-PAGE gel (Fig. 3A, lane 1). The incorporation of caged cysteine to PCNA was confirmed by mass spectrometry analysis. The determined molecular weight (MW) of 31,228 Da agreed well with the calculated MW of 31,231 Da (Fig. S1 ESI†).
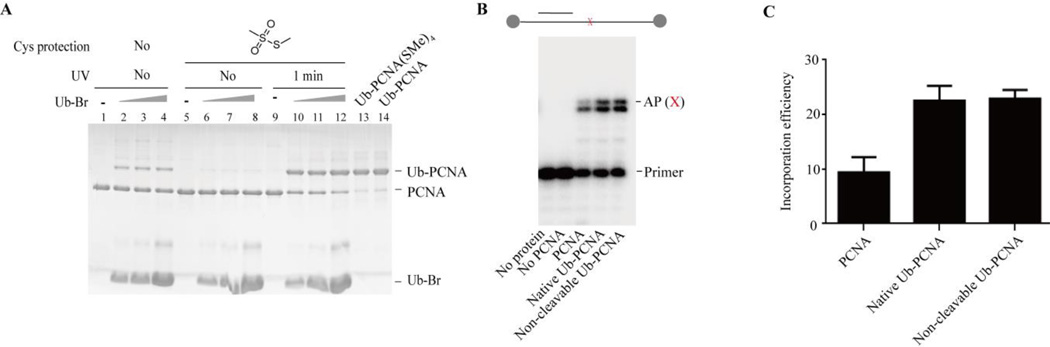
Figure 3.
Generation of non-cleavableUb-PCNA using MMTS to reversibly protect native cysteines. (A) caged Cys-PCNA, caged Cys-PCNA(SMe)4 or caged Cys-PCNA(SMe)4 following 1 min UV irradiation was incubated with increasing concentrations of Ub-Br respectively at room temperature for 2 hours. The products were resolved on a 20% SDS-PAGE gel and stained with Coomassie blue. Ub-PCNA(SMe)4 was purified using Q column and then treated with 100 mM DTT at room temperature for 4 hours to generate the final Ub-PCNA product. (B) non-cleavable Ub-PCNA stimulates TLS of abasic site lesion of Polη. The two circles indicate the biotin–streptavidin blocks. X indicates the abasic site. A representative 15% denaturing PAGE gel showing the DNA synthesis opposite the abasic site by Polη in the presence of 50 nM concentration of each of the PCNA species as indicated using the above abasic site-containing DNA. (C) Quantification of the incorporation efficiency opposite the abasic site by increasing concentration of Polη with various PCNA species.
Without protection of the native cysteines on PCNA, multiple ubiquitinated PCNA bands were observed after the reaction of caged Cys-PCNA with Ub-Br (Fig. 3A, lane2–4), indicating nonspecific ubiquitination of PCNA. To achieve specific ubiquitination, MMTS was used to protect the native cysteines on PCNA. MMTS reacts specifically with cysteine to form dithiomethane32. The small size of the methylsulfanyl group on cysteine is desirable in maintaining the proper folding and structure of the target protein. After treatment with 100 equivalents of MMTS at room temperature for 1 hour, the MW of caged Cys-PCNA increased from 31,228 Da to 31,416 Da (Fig. S2 ESI†). The increase of 188 Da indicated that all four cysteines on PCNA were modified by methylsulfanyl groups to form caged Cys-PCNA(SMe)4. Cysteine modification by the methylsulfanyl group prevented the conjugation of Ub-Br to PCNA as there were no observed adduct bands after incubation of caged Cys-PCNA (SMe)4 with increasing concentration of Ub-Br (Figure 3A, lane 5–8).
To investigate the stability of dithiomethane adduct under UV irradiation used for the deprotection of the caging group on cysteine 164, the following control experiment was done. The wild-type PCNA (wt-PCNA) was purified and all four native cysteines were protected with the methylsulfanyl groups after incubation with MMTS (Fig. S3A–B ESI†). Following irradiation of 1 minute at a wavelength of 365 nm, the MW of wt-PCNA(SMe)4 remained unchanged (Fig. S3C ESI†). In addition, Ub-Br did not react with the UV irradiated wt-PCNA(SMe)4 given that no clear Ub-PCNA adducuts were observed (Fig. S3D ESI†). Thus, the dithiomethane adduct is stable under the brief UV irradiation.
Following irradiation of 1 minute at a wavelength of 365 nm, the MW of the caged Cys-PCNA(SMe)4 was decreased from 31,416 Da to 31,224 Da (Fig. S4 ESI†). The removal of the caging group was close to completion. To avoid the intermolecular disulfide bond exchange between the exposed Cys 164 and the dithiomethane on PCNA, Ub-Br was added to the PCNA solution immediately following the UV irradiation. A single Ub-PCNA(SMe)4 band was observed with approximately 80% conversion (Fig. 3A, lane 9–12). After a purification step on Q column, Ub-PCNA(SMe)4 with approximately 90% purity was achieved (Fig 3A, lane 13). The conjugation of ubiquitin to PCNA was confirmed by LC-MS. The determined MW of 39,798 Da agreed well with the calculated MW of 39,795 Da (Fig. S5A ESI†). In the final step, the Ub-PCNA(SMe)4 product was treated with 100 mM DTT for 4 hours at room temperature to remove the methylsulfanyl groups on the native PCNA cysteines. The MW of Ub-PCNA(SMe)4 was decreased from 39,798 Da to 39,608 Da (Fig. S5B ESI†). The 190 Da difference indicated a complete deprotection of the four native cysteines. The Ub-PCNA remained intact during the DTT treatment because of the non-cleavable linker (Fig 3A, lane 14). The position of modification on PCNA was confirmed by tryptic digestion of the Ub-PCNA product and MS/MS analysis of the PCNA peptide with a glycine connected through the non-cleavable linker (Fig. S6 ESI†). Furthermore, the Ub-PCNA product remained intact after incubation with yeast cell lysates at room temperature for 4 hours (Fig. S7 ESI†).Thus, the Ub-PCNA with non-cleavable linker can be applied to both the reconstituted and cell lysate-based assays.
We next investigated the activity of the non-cleavable Ub-PCNA and compared it to that of the native Ub-PCNA. The non-cleavable Ub-PCNA stimulated the ATPase activity of RFC with a similar efficiency as the native Ub-PCNA (Fig. S8 ESI†). We also assayed the translesion synthesis (TLS) past an abasic site by Polη in the presence of non-cleavable Ub-PCNA or native Ub-PCNA. Agreeing with previous result29, the non-cleavable Ub-PCNA promoted TLS opposite the abasic site by Polη (Fig. 3B). Importantly, similar deoxyribonucleotide incorporation efficiency opposite the abasic lesion site was observed comparing the non-cleavable Ub-PCNA and the native Ub-PCNA(Fig. 3C). Thus, the non-cleavable Ub-PCNA retained a normal function as the native Ub-PCNA.
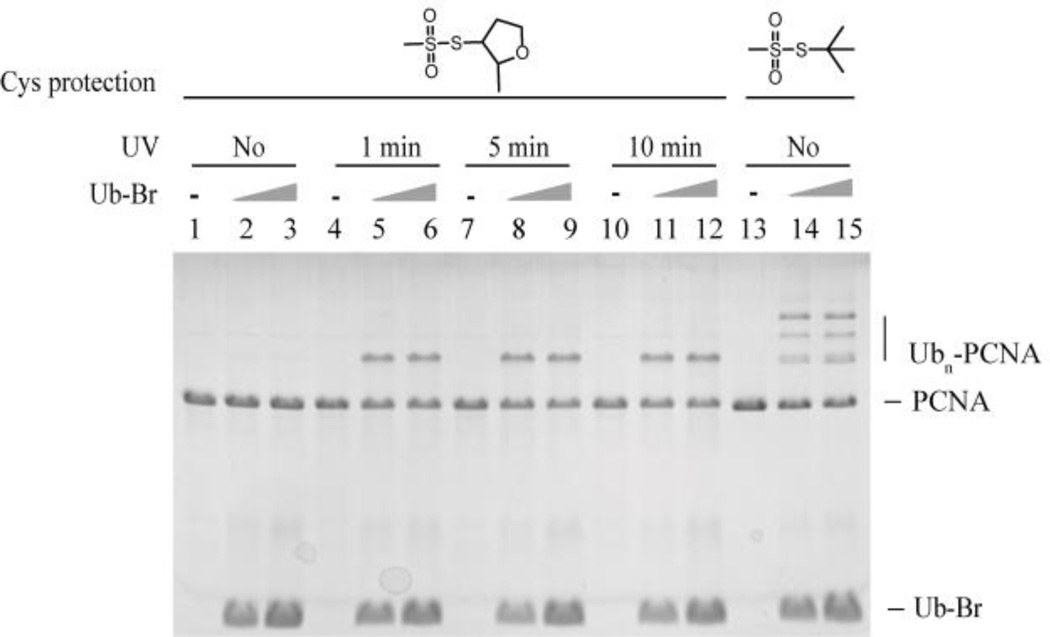
In addition to MMTS, we also assessed 2-methyl tetra-hydrofuran-methanethiosulfonate (MTHFMTS) and tert-butyl-methanethiosulfonate (TBMTS) as the protection groups of the native cysteines on PCNA (Fig. 4). 2-methyl tetra-hydrofuran and tert-butyl systems are bulkier than methyl group. MTHFMTS was synthesized following the protocol used to produce TBMTS33. After treatment with 100 eqv. MTHFMTS at room temperature for 1 hour, the MW of caged Cys-PCNA was increased from 31,228 Da to 31,686 Da (Fig. S9A ESI†). The increase of 458 Da indicated that all four cysteines on PCNA were modified by 2-methyltetrahydrofuran-3-sulfanyl (SMeTHF). Ub-Br did not react with the SMeTHF protected PCNA (Fig. 4, lane 1–3). However, only around 20% ligation product was observed following 1 min UV irradiation (Fig. 4, lane 4–6), and longer UV irrdiation did not improve the ligation efficiency (Fig. 4, lane 7–12). Thus, cysteine protection by SMeTHF likely inhibited the deprotection of the caged cysteine. This was confirmed by mass spectrometry and approximately 30% of the photocaging group was removed after 1 minute UV irradiation of caged Cys-PCNA(SMeTHF)4 (Fig. S9B ESI†).
Figure 4.
Protection of native cysteines on PCNA using MTHFMTS and TBMTS. Caged Cys-PCNA(SMeTHF)4, UV irradiated caged Cys-PCNA(SMeTHF)4 or caged Cys-PCNA(StBu) was incubated with increasing concentration of Ub-Br at room temperature for 2 hours. The products were resolved on 20% SDS-PAGE gel and stained with Coomassie blue.
Unlike MMTS and MTHFMTS, after treatment with 100 eqv. TBMTS at room temperature for 1 hour, the MW of caged Cys-PCNA increased from 31,228 Da to 31,315 Da (Fig. S10 ESI†). The increase of 87 Da indicated that only one of the four cysteines on PCNA was modified by tert-butylthio (StBu). MS/MS analysis indicated that Cys81 was modified (Fig. S11 ESI†). It is possible that due to the large size of the tert-butyl group, only the more exposed C81 on PCNA was modified. The incomplete protection of the native cysteines was evident given that the protected PCNA still reacted with Ub-Br (Fig. 4, lane 13–15).
Conclusion
We reported a new method to generate non-cleavable Ub-PCNA while preserving the native cysteines on PCNA. A caged cysteine was genetically incorporated into PCNA at the ubiquitination site. Following the photo-deprotection under a mild condition, site-specific monoubiquitination of PCNA was achieved using a ubiquitin modified with a non-cleavable linker. This method requires the protection of all native PCNA surface cysteines with MMTS and the protection groups can be removed following the ligation reaction by DTT treatment. The obtained Ub-PCNA with the non-cleavable linker is as active as its native counterpart. It is also stable in buffers containing high concentration of reducing agent and in cell lysates. In addition to PCNA ubiquitination, this method may be adapted for chemical ubiquitination of other proteins. Moreover, this strategy can also be used for site-specific modification of a target protein at a specific site through sulfhydryl chemistry while preserving the native cysteines in the target protein.
Supplementary Material
Acknowledgments
We thank Jason Chin (MRC Laboratory of Molecular Biology) for the generous gift of pBK-PCC2RS and psfGFP150TAGPylT-His6. We thank Lajos Haraska (Hungarian Academy of Sciences) for the generous gift of native Ub-PCNA. We thank Colin Thorpe for useful discussions. This work was supported in part by grants from the U.S. National Science Foundation (MCB-0953764) and National Institutes of Health (R21NS085509) to Z.Zhuang. This publication was made possible by the Delaware COBRE program through a grant from the National Institute of General Medical Sciences (1 P30 GM110758-01) from the National Institutes of Health.
References
- 1.Komander D, Rape M. Annual review of biochemistry. 2012;81:203. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 2.Spasser L, Brik A. Angew Chem Int Ed Engl. 2012;51:6840. doi: 10.1002/anie.201200020. [DOI] [PubMed] [Google Scholar]
- 3.Strieter ER, Korasick DA. ACS chemical biology. 2012;7:52. doi: 10.1021/cb2004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haracska L, Unk I, Prakash L, Prakash S. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6477. doi: 10.1073/pnas.0510924103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGinty RK, Kim J, Chatterjee C, Roeder RG, Muir TW. Nature. 2008;453:812. doi: 10.1038/nature06906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGinty RK, Kohn M, Chatterjee C, Chiang KP, Pratt MR, Muir TW. ACS chemical biology. 2009;4:958. doi: 10.1021/cb9002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Fekner T, Ottesen JJ, Chan MK. Angew Chem Int Ed Engl. 2009;48:9184. doi: 10.1002/anie.200904472. [DOI] [PubMed] [Google Scholar]
- 8.Weikart ND, Mootz HD. Chembiochem : a European journal of chemical biology. 2010;11:774. doi: 10.1002/cbic.200900738. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee C, McGinty RK, Fierz B, Muir TW. Nature chemical biology. 2010;6:267. doi: 10.1038/nchembio.315. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Ai Y, Wang J, Haracska L, Zhuang Z. Nature chemical biology. 2010;6:270. doi: 10.1038/nchembio.316. [DOI] [PubMed] [Google Scholar]
- 11.Freudenthal BD, Gakhar L, Ramaswamy S, Washington MT. Nature structural & molecular biology. 2010;17:479. doi: 10.1038/nsmb.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haj-Yahya M, Ajish Kumar KS, Erlich LA, Brik A. Biopolymers. 2010;94:504. doi: 10.1002/bip.21384. [DOI] [PubMed] [Google Scholar]
- 13.Fierz B, Chatterjee C, McGinty RK, Bar-Dagan M, Raleigh DP, Muir TW. Nature chemical biology. 2011;7:113. doi: 10.1038/nchembio.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virdee S, Kapadnis PB, Elliott T, Lang K, Madrzak J, Nguyen DP, Riechmann L, Chin JW. Journal of the American Chemical Society. 2011;133:10708. doi: 10.1021/ja202799r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eger S, Castrec B, Hubscher U, Scheffner M, Rubini M, Marx A. Chembiochem : a European journal of chemical biology. 2011;12:2807. doi: 10.1002/cbic.201100444. [DOI] [PubMed] [Google Scholar]
- 16.Meier F, Abeywardana T, Dhall A, Marotta NP, Varkey J, Langen R, Chatterjee C, Pratt MR. Journal of the American Chemical Society. 2012;134:5468. doi: 10.1021/ja300094r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Zhang S, Lin SH, Wang X, Wu L, Lee EY, Lee MY. Cell Cycle. 2012;11:2128. doi: 10.4161/cc.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keren-Kaplan T, Prag G. Acta crystallographica. Section F, Structural biology and crystallization communications. 2012;68:1120. doi: 10.1107/S1744309112034331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hibbert RG, Sixma TK. The Journal of biological chemistry. 2012;287:39216. doi: 10.1074/jbc.M112.389890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker R, Lewis SM, Sasaki AT, Wilkerson EM, Locasale JW, Cantley LC, Kuhlman B, Dohlman HG, Campbell SL. Nature structural & molecular biology. 2013;20:46. doi: 10.1038/nsmb.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faggiano S, Menon RP, Kelly GP, McCormick J, Todi SV, Scaglione KM, Paulson HL, Pastore A. FEBS open bio. 2013;3:453. doi: 10.1016/j.fob.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh RK, Sundar A, Fushman D. Angew Chem Int Ed Engl. 2014;53:6120. doi: 10.1002/anie.201402642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan RE, Chudasama V, Moody P, Smith ME, Caddick S. Organic & biomolecular chemistry. 2015;13:4165. doi: 10.1039/c5ob00130g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madrzak J, Fiedler M, Johnson CM, Ewan R, Knebel A, Bienz M, Chin JW. Nature communications. 2015;6:6718. doi: 10.1038/ncomms7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seenaiah M, Jbara M, Mali SM, Brik A. Angew Chem Int Ed Engl. 2015;54:12374. doi: 10.1002/anie.201503309. [DOI] [PubMed] [Google Scholar]
- 26.Lewis YE, Abeywardana T, Lin YH, Galesic A, Pratt MR. ACS chemical biology. 2016 doi: 10.1021/acschembio.5b01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haj-Yahya M, Fauvet B, Herman-Bachinsky Y, Hejjaoui M, Bavikar SN, Karthikeyan SV, Ciechanover A, Lashuel HA, Brik A. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17726. doi: 10.1073/pnas.1315654110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemantha HP, Bavikar SN, Herman-Bachinsky Y, Haj-Yahya N, Bondalapati S, Ciechanover A, Brik A. Journal of the American Chemical Society. 2014;136:2665. doi: 10.1021/ja412594d. [DOI] [PubMed] [Google Scholar]
- 29.Yang K, Gong P, Gokhale P, Zhuang Z. ACS chemical biology. 2014;9:1685. doi: 10.1021/cb500133k. [DOI] [PubMed] [Google Scholar]
- 30.Rosner D, Schneider T, Schneider D, Scheffner M, Marx A. Nature protocols. 2015;10:1594. doi: 10.1038/nprot.2015.106. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen DP, Mahesh M, Elsasser SJ, Hancock SM, Uttamapinant C, Chin JW. Journal of the American Chemical Society. 2014;136:2240. doi: 10.1021/ja412191m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karala AR, Ruddock LW. Antioxid Redox Signal. 2007;9:527. doi: 10.1089/ars.2006.1473. [DOI] [PubMed] [Google Scholar]
- 33.Desbenoit N, Galardon E, Frapart Y, Tomas A, Artaud I. Inorganic chemistry. 2010;49:8637. doi: 10.1021/ic101148c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.