Figure 1.
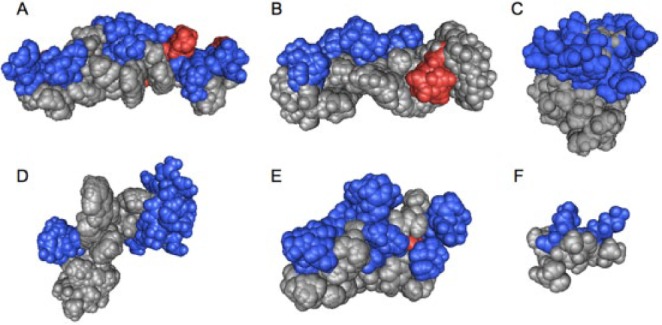
Amphipathic 3-dimensional structures of cationic antimicrobial peptides: (A) cathelicidin LL-37 (residues 2 to 30; pdb 2K6O), (B) magainin 2 (2MAG), (C) lactoferrin (residues 1 to 11; 1XV4), (D) indolicidin (1G89), (E) human defensin 5 (2LXZ), (F) helical model of GL13K (residues 5 to 13). The positively (blue) and negatively (red) charged residues are highlighted. The structures, except that of defensin 5, were obtained in the presence of detergent micelles by solution nuclear magnetic resonance spectroscopy. All low-energy conformations of the PDB files are included in the image to obtain a better view of the full conformational space. Notably, the peptides exhibit different degrees of hydrophobicity, hydrophobic moment, and amphipathicity and consequently differ in their membrane interactions. Space-filling models of cationic amphipathic peptides were created with the Cn3D software (Wang et al. 2000).
