Figure 2.
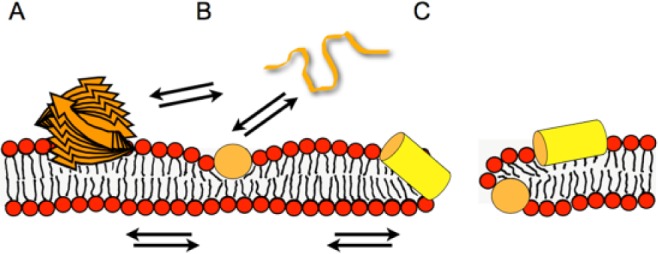
Models illustrating the membrane interactions of antimicrobial peptides. The peptide (orange and yellow) is illustrated as a stack of arrows (beta-sheet aggregate) (A), a random coil string (B), or a cylinder representing a helical structure (yellow, side view; orange, end-on view; B, C). Whereas at low peptide density, the soft membranes adjust to maintain the membrane integrity (B), at higher local peptide concentrations, the peptide-imposed curvature strain on the lipid bilayer causes transient openings (C). Multiple equilibria govern the membrane-association processes, including peptide in aqueous solution (random coil; B) ⇌ amphipathic monomers at the membrane surface ⇌ peptide-lipid supramolecular assemblies causing membrane openings. Additionally, depending on the peptide, beta-sheet membrane oligomers (A) or aggregated peptide structures form in solution. Panel B represents the adaptation of soft membranes to external stimuli (SMART model: Soft Membranes Adapt and Respond, also Transiently, in the presence of antimicrobial peptides), the alignment along the surface being a preliminary state to the carpet model, where a high peptide density causes membrane lysis. At intermediate peptide concentrations, transient openings form that have a toroidal shape made of lipids and peptide. To add another layer of complexity, cationic amphipathic AMPs have recently been shown to arrange in mesophase structures along the surface of charged lipid bilayers (Aisenbrey and Bechinger 2014).
