Abstract
Background:
Arthroscopic repair of large to massive rotator cuff tears commonly retear. To improve healing rates, a number of different approaches have been utilized, including the use of grafts, which may enhance the biomechanical and biologic aspects of the repair construct. However, the outcomes after the use of grafts are diverse.
Purpose:
To systematically review the literature for large to massive rotator cuff tears to determine whether the use of grafts generally provides superior tendon healing and clinical outcomes to the repairs without grafts.
Study Design:
Systematic review; Level of evidence, 3.
Methods:
A systematic review of the literature was performed. Clinical studies comparing the repairs with (graft group) and without grafts (control group) were included and analyzed. The primary outcome was tendon healing on either magnetic resonance imaging or ultrasound. The secondary outcome measures included visual analog scale for pain, University of California at Los Angles (UCLA) score, and forward elevation range. Differences between groups in all outcome measures were statistically analyzed.
Results:
Six comparative studies (level of evidence 2 or 3) with 13 study groups were included. A total of 242 repairs in the graft group (mean age, 62.5 ± 4.6 years) and 185 repairs in the control group (mean age, 62.5 ± 5.0 years) were analyzed. The graft types utilized included autograft (fascia lata) in 1 study, allograft (human dermis) in 2 studies, xenograft (bovine pericardium, porcine small intestine submucosa) in 2 studies, synthetic graft (polypropylene) in 1 study, and a combination of autograft (the long head of biceps) and synthetic graft (polypropylene) in 1 study. The overall mean follow-up time was 28.4 ± 9.0 months. When 1 or 2 studies/study groups were excluded due to practical or statistical reasons, the graft group demonstrated significantly improved healing (odds ratio, 2.48; 95% CI, 1.58-3.90; P < .0001) and all clinical outcome measures at final follow-up (P ≤ .02).
Conclusion:
The use of grafts generally provides superior tendon healing and clinical outcomes compared to repairs without grafts, except for some specific graft types (eg, porcine small intestine submucosa, bovine pericardium). Further investigations are required to determine the benefits of the use of grafts.
Keywords: rotator cuff, repair, graft, tendon healing
Rotator cuff tears are a common pathology causing shoulder pain in the adult. Even a decade ago, more than 75,000 surgical repairs of the rotator cuff were performed annually in the United States with an increasing trend.27,41 Despite advancements in surgical technique and technology for rotator cuff repair, high failure rates (eg, retearing or nonhealing of the repairs) are still a concern.17 While tendon healing may be affected by multiple factors (age, smoking, tear characteristics, repair techniques, postoperative rehabilitation protocols), tear size is one of the most critical factors. In particular, large to massive rotator cuff tears are particularly challenging to the shoulder surgeon due to their inferior healing rates when compared with smaller tears and their relatively high prevalence (up to 40% of all the rotator cuff tears).3,17,20,40
For any size of rotator cuff tear requiring surgical intervention, primary repair is the ideal option. However, in larger tears, this may not be achievable. In this situation, other salvage-type procedures may be viable options, including debridement,18 biceps tenodesis or tenotomy,4 partial repair,7 superior capsular reconstruction,31 or even reverse shoulder arthroplasty.22 These procedures can provide fair to good outcomes,3,20 particularly in older, less demanding patients. However, in younger active patients, grafts in combination with partial or full repair have been used in an attempt to reinforce the tendon tissue and distribute the mechanical load through the repair construct.3,14,20 Furthermore, grafts may improve the biologic milieu of the rotator cuff repair, functioning as a scaffold and inducing cellular migration and matrix production.40
The use of a graft during rotator cuff repair was first reported by Neviaser et al34 in 1978. In a series of 16 patients, allograft rotator cuff tendons were utilized to bridge the gap between the retracted tendon edge and the bone of irreparable rotator cuff tears.34 Since then, a variety of grafts (autograft, allograft, xenograft, and synthetic graft) have been introduced and applied with fair to good tendon healing and clinical outcomes.14
While some clinical studies have compared the outcomes after rotator cuff repair with and without the use of grafts, the results remain diverse.2,9,11,19,26,33,42 Furthermore, only a few studies have compared the use of grafts in a prospective randomized fashion. Therefore, the purpose of the study was to systematically review the literature to determine whether the use of grafts improves tendon healing and clinical outcomes of rotator cuff repair for large to massive rotator cuff tears.
Methods
Systematic Review for Meta-analysis
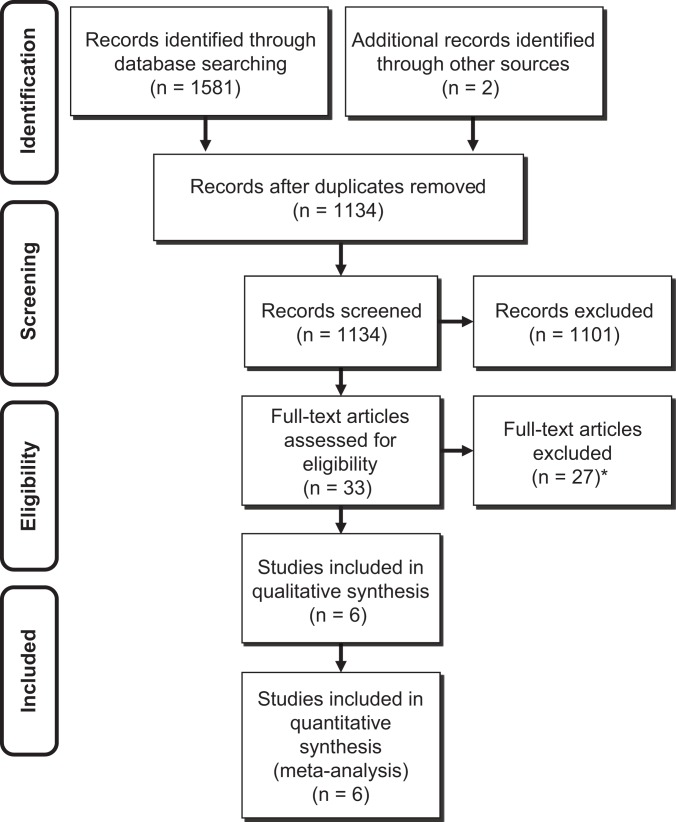
This systematic literature review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and checklist32 (Figure 1). Two independent reviewers conducted a thorough literature search of the following databases: PubMed, MEDLINE, Embase, and Cochrane Library. The search terms included the following: rotator cuff, repair, graft, patch, scaffold, augmentation, reinforcement, bridging, interposition, replacement, and spanning.
Figure 1.
Systemic review algorithm using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. *Twenty-two articles were case series with no comparison group, 2 articles reported only surgical techniques, 1 article compared groups depending on the degree of fatty infiltration with no control group, 1 article reported in non–English language, 1 article did not perform postoperative imaging evaluation (magnetic resonance imaging or ultrasound).
Studies were selected and systematically reviewed according to the following inclusion criteria: (1) clinical study comparing rotator cuff repair with (graft group) or without graft (control group), (2) either an open or arthroscopic procedure or both, (3) use of grafts as either augmentation or bridging, (4) tendon healing assessed by magnetic resonance imaging (MRI) or ultrasound (US) postoperatively for at least 80% of cases, and (5) English language.
Studies were excluded if they were: (1) nonclinical (eg, cadaver, animal, basic science, biomechanical) studies, (2) scientific meeting abstracts/proceedings, (3) perception-based studies, (4) review or meta-analysis articles, (5) case series or cohort studies without control group (ie, repair without grafts), and (6) not written in English.
The search was conducted by 2 independent investigators separately, each reviewing the abstract of each publication, and the data were extracted from each relevant article. The final literature search was performed in August 2015. All references of included studies were cross-referenced to avoid omitting relevant studies that were originally not included. If there was disagreement regarding the inclusion of a study, the final decision was ultimately made by the senior author (I.K.Y.L.). For studies where duplicate patient populations were reported, only the most recent publication was used for data extraction and analysis.
Quality Assessment
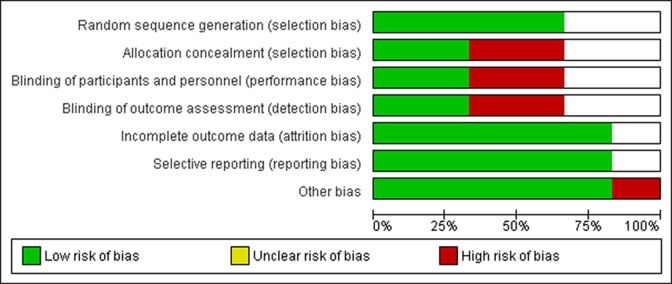
The evidence levels of the included studies were determined using the guide outlined by the Oxford Centre for Evidence Based Medicine.35 The quality of studies was assessed following the Modified Coleman Methodology Score (MCMS).12
Outcome Measures
Tendon healing on postoperative MRI or US was defined as the primary outcome. Healed or intact repairs were classified as “healed” tears. However, partially healed, partially retorn, retorn, or nonhealed tears were all classified as “retorn.” Secondary outcomes included visual analog scale (VAS) for pain, the University of California at Los Angles (UCLA) score, and range of motion in forward elevation (FE). Reported complications were also extracted and assessed while retears were not included as complications and reported separately.
Statistical Analysis
The data were synthesized using the software Review Manager 5.3 (Cochrane Informatics and Knowledge Management Department; http://tech.cochrane.org/home). Random-effects models were used if the chi-square test for heterogeneity failed with P < .05; otherwise, fixed-effects models were used. For each outcome, we produced forest plots along with numeric estimates of overall effects along with 95% CIs, and funnel plots were used to assess publication bias. In addition, a risk-of-bias assessment graph was produced for the included studies using the Cochrane Collaborations tool.24
Results
Study Demographics
A total of 6 studies with 13 study groups were included (Table 1). The levels of evidence were between 2 and 3. The included data were taken from 2 prospective randomized trials, 2 prospective nonrandomized study, and 2 retrospective cohort studies. The overall mean MCMS was 55.8 (fair quality). The risk-of-bias assessment is summarized in Figure 2. A total of 242 repairs from 7 study groups in the graft group and 185 repairs from 6 study groups in the control group were analyzed. The mean age at surgery was 62.5 years for both groups, and the mean follow-up was 29.5 months for the graft group and 28.3 months for the control group (Table 1). The surgical approach (open, arthroscopy), surgical indication (augmentation, bridging), type and source of graft, and product name are all provided in Table 1.
TABLE 1.
List of Studies Includeda
| Graft Information | No. of Repairs | Mean Age, y | Mean | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Study Type | Level of | Surgical | Source | Follow-up, | ||||||||
| (Year) | Journal | and Design | Evidence | MCMS | Approach | Procedure | Type | (Product Name) | Graft | Control | Graft | Control | mo |
| Gilot et al19 (2015) | Arthroscopy | Prospective cohort | 3 | 47 | Arthroscopy | Augmentation | Allograft | Human dermal (Arthroflex) | 20 | 15 | 58.9 | 62.0 | 24.9 |
| Vitali et al42 (2015) | Tech Hand Up Extrem Surg | Retrospective cohort | 3 | 52 | Open | Combination (bridging + augmentation) | Autograft + synthetic | Biceps + polypropylene (Angiologica) | 60 | 60 | 66.0 | 67.3 | 36 |
| Ciampi et al11 (2014) | Am J Sports Med | Retrospective cohort | 3 | 57 | Open | Augmentation | Xenograft | Bovine pericardium (Tutopatch) | 49 | 51 | 66.5 | 67.1 | 36 |
| Synthetic | Polypropylene (Angiologica) | 52 | 66.2 | ||||||||||
| Mori et al33 (2013) | Arthroscopy | Retrospective cohort | 3 | 53 | Arthroscopy | Bridging | Autograft | Fascia lata | 24 | 24 | 65.9 | 65.4 | 35.6 |
| Barber et al2 (2012) | Arthroscopy | Prospective RCT | 2 | 74 | Arthroscopy | Augmentation | Allograft | Human dermal (Graftjacket) | 22 | 20 | 56.0 | 56.0 | 24 |
| Iannotti et al26 (2006) | J Bone Joint Surg Am | Prospective RCT | 2 | 52 | Open | Augmentation | Xenograft | Porcine intestine submucosa (Restore) | 15 | 15 | 58.0 | 57.0 | 14 |
aMCMS, modified Coleman Methodology Score; RCT, randomized controlled trial.
Figure 2.
Summary of risk-of-bias assessment.
It should be noted that the study by Iannotti et al26 demonstrated a significantly poor healing rate (26.7%) in addition to a number of extensive inflammatory reactions when using a porcine small intestinal submucosal graft. In their study, healing rate was the only measure and no other outcome measures were available. No other study in our meta-analysis utilized a similar graft.
Furthermore, Ciampi et al11 reported on a nonrandomized, triple-armed study of patients utilizing grafts including 2 study groups and 1 control group. The patients received either a bovine pericardium graft, a polypropylene synthetic graft, or no graft as a control, with reported healing rates of 49.0%, 82.7%, and 58.8%, respectively. This study, having 2 study groups, made statistical calculation technically challenging, particularly for the clinical outcome measures with continuous values.
Tendon Healing
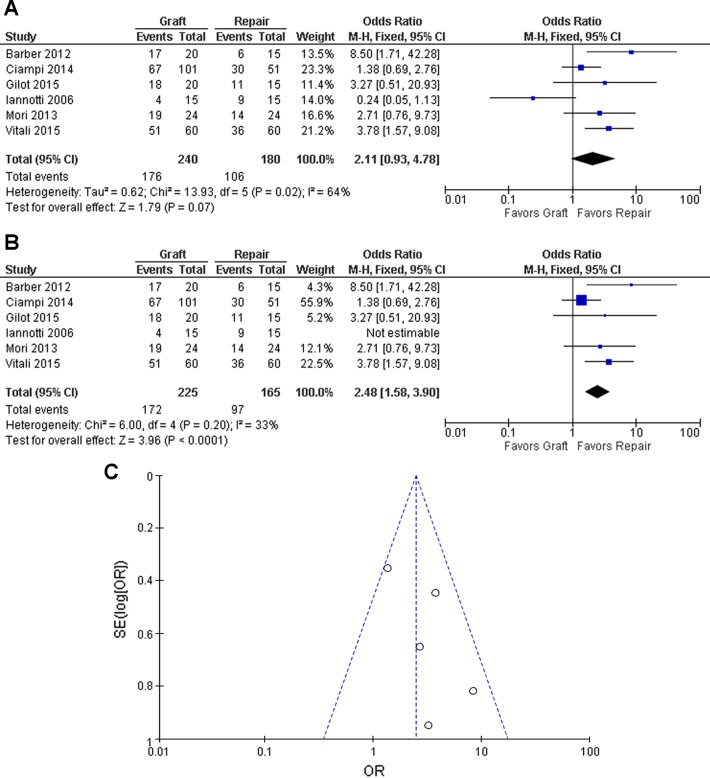
Tendon healing was evaluated either on MRI or US in 240 of 242 repairs in the graft group and 180 of 185 repairs of the control group (Figure 3A). In this analysis, the healing rates of the 2 different graft groups (xenograft group, synthetic graft group) in the study by Ciampi et al11 were both combined as 1 study group. Because of relatively high heterogeneity (P = .02, I2 = 64%), a random-effects model was applied. Overall, there was a trend that the graft group had a greater healing rate than the control group (odds ratio [OR], 2.11; 95% CI, 0.93-4.78; P = .07) (Figure 3A). However, when the study by Iannotti et al26 was further excluded due to the nature of adverse results and reactions of the specific graft, the overall healing rate became statistically significant (OR, 2.48; 95% CI, 1.58-3.90; P < .0001) with less heterogeneity (P =.20, I2 = 33%) where a fixed-effects model was applied (Figure 3, B and C).
Figure 3.
Odds ratio of tendon healing: (A) including Iannotti et al26 and (B) excluding Iannotti et al26 with (C) corresponding funnel plot. The horizontal and vertical axes represent the odds ratio (OR) of tendon healing and the standard error of log(OR), respectively. The dots from top to bottom represent the studies of Ciampi et al,11 Vitali et al42, Mori et al33, Barber et al2, and Gilot et al19, respectively.
Complications
In the graft group, 3 sterile inflammatory reactions, 1 episode of bursitis, and 1 superficial infection were reported as postoperative complications. All cases of sterile inflammatory reaction were reported in 1 study26 where porcine small intestine submucosa was utilized as the graft, 1 of which required additional surgery. In the control group, cellulitis (n = 2), bursitis (n = 1), fibrosis (n = 1), and biceps rupture (n = 1) were reported, and no additional surgery was performed.
Clinical Outcomes Measures
Five study groups in the graft group and 4 study groups in the control group assessed VAS for pain; the UCLA score and FE were evaluated in 4 study groups in the graft group and 3 study groups in the control group. Given that the study by Ciampi et al11 had 2 different graft groups and there was no technically valid statistical method to combine both groups for these continuous measures, 2 alternative sets of analyses were produced based on the graft utilized (xenograft and synthetic graft).
Preoperatively, there was no baseline mean difference between the control group and the graft group for VAS, UCLA score, and FE (P = .28 or higher), even when the xenograft group or synthetic group was included from the study by Ciampi et al.11 The data produced homogeneous results (P ≥ .55, I2 = 0%); hence, fixed-effects models were used for all the measures. Similar results were observed when analyses were performed for tendon healing with the 2 alternative sets (xenograft group or synthetic group) from the study by Ciampi et al.11
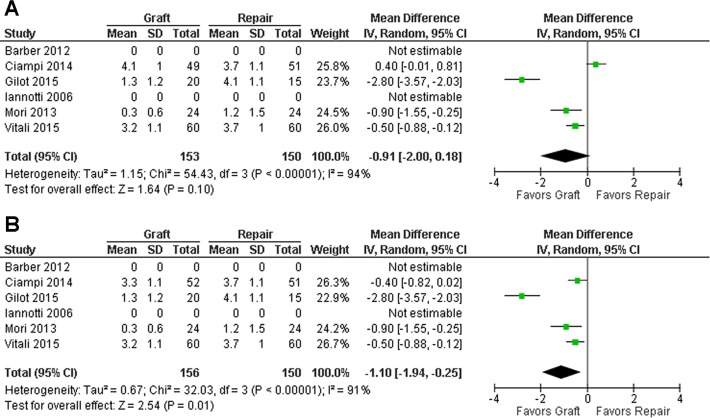
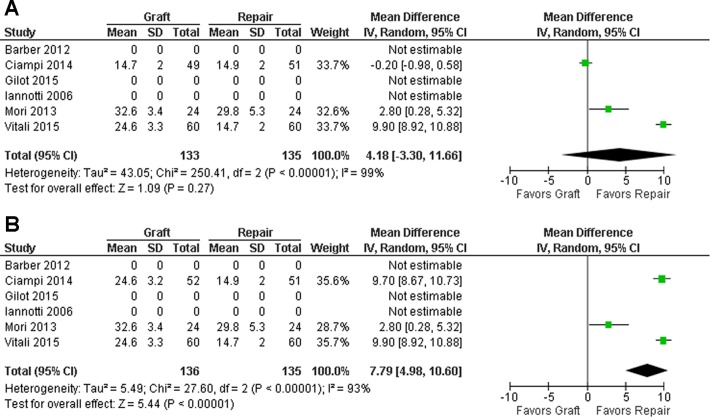
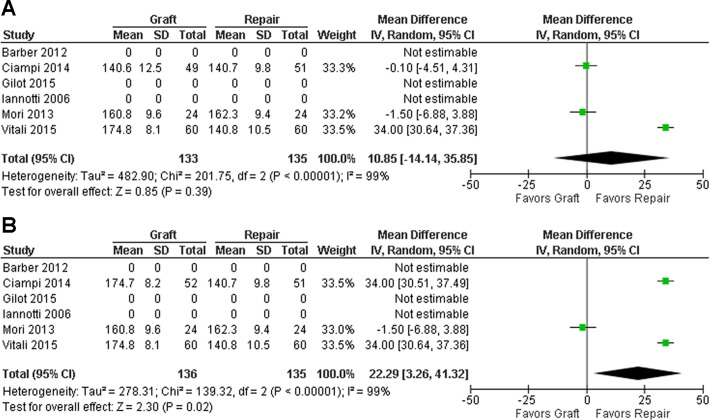
Postoperatively, both control and graft groups showed improvement postoperatively in all the 3 measures (Figures 4 –6). For all measures, there was no significant difference between the groups detected when the xenograft group from Ciampi et al11 was utilized (Figures 4A, 5A, and 6A), whereas the graft group demonstrated significantly superior outcomes in the graft group when the synthetic group of Ciampi et al11 was chosen (P = .02 or lower) (Figures 4B, 5B, and 6B). Because of high heterogeneity, random-effects models were applied to all analyses.
Figure 4.
Mean difference for visual analog scale at follow-up. The figures show the results including the (A) xenograft and (B) synthetic graft study groups from the study by Ciampi et al.11
Figure 5.
Mean difference of University of California–Los Angeles scores at follow-up. The figures show the results including the (A) xenograft and (B) synthetic graft study groups from the study by Ciampi et al.11
Figure 6.
Mean difference of forward elevation range at follow-up. The figures show the results including the study group of (A) xenograft and (B) synthetic graft from Ciampi et al.11
Discussion
Anatomic healing of the torn tendon to the bone is a primary principle of rotator cuff surgery. Even though tendon integrity after rotator cuff repair may not necessarily correlate with clinical outcomes,10,17,37,46 patients in general are as good if not better when the repaired tendon heals.29,39 In an attempt to enhance healing, a number of studies have reported biological augmentation of rotator cuff repair (eg, platelet-rich fibrin), although no study has clearly demonstrated improved healing or clinical outcomes compared with the control group.8,44,47 In contrast, the rationale for the use of grafts is to enhance not only the biological aspect but also the biomechanical strength of the repair construct.
In the current study, the repair of large to massive rotator cuff tears utilizing grafts led to significantly diverse outcomes. In particular, the studies by Iannotti et al26 and Ciampi et al11 demonstrated that not all grafts have consistent outcomes. When all groups were included, there was a trend toward superior tendon healing with grafts, but no statistical significance was detected unless the study by Iannotti et al26 was excluded. Similarly, while there appeared to be a tendency toward superior clinical outcomes (eg, VAS, UCLA score, FE) with the use of grafts, statistical significance was only detected when the synthetic graft study group rather than the xenograft study group from Ciampi et al11 was included. Therefore, grafts provided statistically improved healing and clinical outcomes only when exclusion of a study26 or the choice of study group11 for practical (eg, adverse effects of a porcine small intestine submucosa graft) or statistical reasons (eg, handling 2 study groups in a study) was performed.
Furthermore, the healing rates were also significantly greater with the use of grafts only when the synthetic group (and not the xenograft group) was included from the study by Ciampi et al,11 regardless of the inclusion or exclusion of the data from Iannotti et al.26 Interestingly, both Iannotti et al26 and Ciampi et al11 utilized xenografts, at least as 1 study group, to reinforce the repair of rotator cuff tear. These xenograft groups both provided significantly poor results, leading to diverse outcomes in the overall graft population. From a statistical perspective, excluding these results from the overall graft group led to a more consistent outcome and improved the results to reach statistical significance. Iannotti et al26 demonstrated an inferior healing rate with the use of porcine small intestine submucosa grafts compared with repair without a graft, including 3 cases of sterile inflammatory reaction. This particular porcine xenograft is now known to have a high risk of inflammatory reaction, which has been reported not only in rotator cuff repair studies but also in other nonorthopaedic uses.26,28,30,36,38,43,45 The use of these grafts for rotator cuff repair is rarely used now and is essentially historical in nature. Therefore, this specific graft material likely compromised their results, which led us to perform additional statistical analysis after exclusion of the study.
On the other hand, Ciampi et al11 reported a significantly lower healing rate after the use of bovine pericardium grafts compared with the synthetic grafts but not significantly lower than repair without grafts. Similar findings were reported for clinical outcome measures. However, as far as we are aware, there have been no other studies reporting significant adverse effects or inferior clinical outcomes after the use of this specific graft material.
These results clearly highlight the importance of not only whether a graft was utilized but also the graft material. However, there are a number of other studies on rotator cuff repair that have utilized xenograft materials demonstrating excellent healing and clinical outcomes.1,21 Therefore, the overall graft material in general (autograft, allograft, xenograft, synthetic) should be considered in addition to the specific graft type (eg, differences in sterilization, cross-linking, composition), as this may affect healing and clinical outcomes.
In the current study, we included only comparative studies with a control group (repair without grafts) in an attempt to ensure a similar population of patients was being evaluated for each group. This was aimed to minimize the effects of other variables related to rotator cuff repair that may affect the outcome, such as age, smoking, and tear characteristics. Despite this, a uniform patient and tear population may have been difficult to achieve. For example, even though all included studies reported the surgical results of large to massive rotator cuff tears, there were variable ways of defining “reparability” of the tears. For example, some studies included only “irreparable” tears, others included tears that were partially reparable, and others reported on only tears that were “reparable.” However, even among “reparable” tears, 1 study accepted a “defect smaller than 1 cm,” another study allowed “retraction less than 2 cm,” and another study defined the tears “fully” reparable. Thus, despite efforts to achieve a uniform population of large to massive tears, clearly the actual tear characteristics (eg, retraction, mobility) may be quite variable, similar to the clinical setting.
Consistent with tear reparability variation, both graft augmentation and bridging indications existed among the studies. Generally, augmentation is primarily performed to reinforce a tear that is fully reparable. In contrast, bridging grafts are used to fill a defect between the torn tendon edge and bone in the setting of an irreparable tear. Accordingly, among the 6 analyzed studies, 4 studies of “reparable” tears applied an augmentation technique, and the other 2 studies reporting “irreparable” tears performed a bridging procedure. Thus, these 2 groups of studies may represent a slightly different patient population. However, this allowed us to form a clinically relevant spectrum of patients to whom grafts may be applied and was the rationale for including only comparative studies with a control group to ensure similar populations in each group.
Recently, Ferguson et al16 published a systematic review limited to graft augmentation of reparable large to massive rotator cuff tears. This article included a wide range of published literature from case series to comparative studies and demonstrated superior function and structural outcome with the use of human dermal allografts when compared with repair without grafts. In contrast, as previously mentioned, we selectively analyzed only comparative studies with a control group and included both augmentation and bridging indications. We believe this allowed us to provide more generalized information with clinical relevance focusing purely on the effect of the graft usage for rotator cuff tears.
In a recent systematic review on the healing rates after rotator cuff repair,23 for rotator cuff tears greater than 3 cm.
The reported healing rates were 52%, 66%, and 69% for single-row, double-row, and suture-bridge techniques, respectively. In the current study, the overall healing rate in the control group (without grafts) was 58.9% (106/180 repairs), which is comparable to these healing rates. Unfortunately, due to the various procedures used for repair in the current study, it was impossible to categorize the repair technique (single-row, double-row, suture-bridge) clearly. While each of the 6 analyzed studies utilized a similar procedure between the graft group and the control group except for the use of graft, repair techniques were different among the studies (single-row in 2 studies, double-row in 1 study, transosseous in 1 study, not specified in 2 studies). Thus, the difference in tendon fixation techniques may have had an effect on the healing rate as well.
Further to the above, Denard et al13 reported in their retrospective study that 85% of massive rotator cuff tears were actually reparable using multiple tendon mobilization techniques, with excellent functional outcomes especially after double-row repair. While the authors did not assess postoperative tendon healing, secure fixation under minimal tension with a wide contact area is likely an important factor, allowing even larger tendon tears to heal. Some authors have hypothesized that tension on the repair construct can lead to retearing of a rotator cuff repair.5,6,15 Thus, a repair of a large to massive rotator cuff despite releases is likely to be under at least some degree of tension irrespective of the use of a graft. A higher tension repair may have led to pain and retearing of the rotator cuff repair.5,6,15,25
There are several limitations to the present study due to multiple factors involving the outcomes of this challenging patient population. First, although we tried to include only higher level of evidence studies (eg, no case series), the comparative studies evaluated had levels of evidence between 2 and 3 and the study designs were different. Furthermore, only 2 studies were prospective randomized control trials. Therefore, there may be inherent biases in patient selection related to the nonrandomized studies. However, all studies appeared to have recruited comparable patient populations, and there were no significant differences in baseline characteristics for the included studies. Second, as explained, there are a multitude of factors that may affect tendon healing and clinical outcomes (eg, patient characteristics, graft material, tear characteristics, graft indication, and fixation technique). While further stratification would have made the groups more uniform, the purpose of this study was to determine whether any generalizable conclusions could be made between rotator cuff repairs with grafts versus without grafts. Unfortunately, due to the limited number of studies, further stratification would have depleted the number of studies or patients making statistical evaluation invalid. Finally, the majority of the studies included only primary repairs (6 of 8; 2 studies not stated), and few or no cases were revision surgeries. Therefore, the current results are only generalizable to primary rotator cuff repair. However, in the clinical setting, grafts may be considered as well during revision rotator cuff repair, particularly when another standard repair technique is unlikely to be successful. Thus, in addition to the current results, the healing rates and outcomes after revision rotator cuff repair with the use of grafts may provide important information for their further utilization.
Conclusion
The use of grafts generally provides superior tendon healing and clinical outcomes to the repairs without grafts, except for some specific graft types. Further investigations are required to determine the beneficial effects of the use of grafts.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1. Badhe SP, Lawrence TM, Smith FD, Lunn PG. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg. 2008;17(1 suppl):35S–39S. [DOI] [PubMed] [Google Scholar]
- 2. Barber FA, Burns JP, Deutsch A, Labbe MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28:8–15. [DOI] [PubMed] [Google Scholar]
- 3. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92:1894–1908. [DOI] [PubMed] [Google Scholar]
- 4. Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89:747–757. [DOI] [PubMed] [Google Scholar]
- 5. Burkhart SS, Diaz Pagan JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13:720–724. [DOI] [PubMed] [Google Scholar]
- 6. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic Loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13:172–176. [DOI] [PubMed] [Google Scholar]
- 7. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10:363–370. [DOI] [PubMed] [Google Scholar]
- 8. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39:258–265. [DOI] [PubMed] [Google Scholar]
- 9. Cho NS, Yi JW, Rhee YG. Arthroscopic biceps augmentation for avoiding undue tension in repair of massive rotator cuff tears. Arthroscopy. 2009;25:183–191. [DOI] [PubMed] [Google Scholar]
- 10. Chung SW, Kim JY, Kim MH, Kim SH, Oh JH. Arthroscopic repair of massive rotator cuff tears: outcome and analysis of factors associated with healing failure or poor postoperative function. Am J Sports Med. 2013;41:1674–1683. [DOI] [PubMed] [Google Scholar]
- 11. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42:1169–1175. [DOI] [PubMed] [Google Scholar]
- 12. Cowan J, Lozano-Calderon S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89:1693–1699. [DOI] [PubMed] [Google Scholar]
- 13. Denard PJ, Jiwani AZ, Ladermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28:909–915. [DOI] [PubMed] [Google Scholar]
- 14. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467–476. [DOI] [PubMed] [Google Scholar]
- 15. Dini AA, Snyder AJ. Rotator cuff repair—the SCOI row method. Medicina Fluminensis. 2015;51:114–126. [Google Scholar]
- 16. Ferguson DP, Lewington MR, Smith TD, Wong IH. Graft utilization in the augmentation of large-to-massive rotator cuff repairs: a systematic review [published online February 4, 2016]. Am J Sports Med. doi:10.1177/0363546515624463. [DOI] [PubMed] [Google Scholar]
- 17. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219–224. [DOI] [PubMed] [Google Scholar]
- 18. Gartsman GM. Massive, irreparable tears of the rotator cuff. Results of operative debridement and subacromial decompression. J Bone Joint Surg Am. 1997;79:715–721. [DOI] [PubMed] [Google Scholar]
- 19. Gilot GJ, Alvarez-Pinzon AM, Barcksdale L, Westerdahl D, Krill M, Peck E. Outcome of large to massive rotator cuff tears repaired with and without extracellular matrix augmentation: a prospective comparative study. Arthroscopy. 2015;31:1459–1465. [DOI] [PubMed] [Google Scholar]
- 20. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24:1493–1505. [DOI] [PubMed] [Google Scholar]
- 21. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41:872–879. [DOI] [PubMed] [Google Scholar]
- 22. Hartzler RU, Steen BM, Hussey MM, et al. Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elbow Surg. 2015;24:1698–1706. [DOI] [PubMed] [Google Scholar]
- 23. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31:2274–2281. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880–890. [DOI] [PubMed] [Google Scholar]
- 26. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238–1244. [DOI] [PubMed] [Google Scholar]
- 27. Iyengar JJ, Samagh SP, Schairer W, Singh G, Valone FH, 3rd, Feeley BT. Current trends in rotator cuff repair: surgical technique, setting, and cost. Arthroscopy. 2014;30:284–288. [DOI] [PubMed] [Google Scholar]
- 28. John TT, Aggarwal N, Singla AK, Santucci RA. Intense inflammatory reaction with porcine small intestine submucosa pubovaginal sling or tape for stress urinary incontinence. Urology. 2008;72:1036–1039. [DOI] [PubMed] [Google Scholar]
- 29. Lambers Heerspink FO, van Raay JJ, Koorevaar RC, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274–1281. [DOI] [PubMed] [Google Scholar]
- 30. Malcarney HL, Bonar F, Murrell GA. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med. 2005;33:907–911. [DOI] [PubMed] [Google Scholar]
- 31. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29:459–470. [DOI] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- 33. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29:1911–1921. [DOI] [PubMed] [Google Scholar]
- 34. Neviaser JSN, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60:681–684. [PubMed] [Google Scholar]
- 35. Obremskey WT, Pappas N, Attallah-Wasif E, Tornetta P, 3rd, Bhandari M. Level of evidence in orthopaedic journals. J Bone Joint Surg Am. 2005;87:2632–2638. [DOI] [PubMed] [Google Scholar]
- 36. Rosario-Quinones F, Magid MS, Yau J, Pawale A, Nguyen K. Tissue reaction to porcine intestinal submucosa (CorMatrix) implants in pediatric cardiac patients: a single-center experience. Ann Thorac Surg. 2015;99:1373–1377. [DOI] [PubMed] [Google Scholar]
- 37. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96:265–271. [DOI] [PubMed] [Google Scholar]
- 38. Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg. 2004;13:538–541. [DOI] [PubMed] [Google Scholar]
- 39. Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26:393–403. [DOI] [PubMed] [Google Scholar]
- 40. Snyder SJ, Arnoczky SP, Bond JL, Dopirak R. Histologic evaluation of a biopsy specimen obtained 3 months after rotator cuff augmentation with GraftJacket Matrix. Arthroscopy. 2009;25:329–333. [DOI] [PubMed] [Google Scholar]
- 41. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16:181–187. [DOI] [PubMed] [Google Scholar]
- 42. Vitali M, Cusumano A, Pedretti A, Naim Rodriguez N, Fraschini G. Employment of synthetic patch with augmentation of the long head of the biceps tendon in irreparable lesions of the rotator cuff: our technique applied to 60 patients. Tech Hand Up Extrem Surg. 2015;19:32–39. [DOI] [PubMed] [Google Scholar]
- 43. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89:786–791. [DOI] [PubMed] [Google Scholar]
- 44. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41:263–270. [DOI] [PubMed] [Google Scholar]
- 45. Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61–67. [DOI] [PubMed] [Google Scholar]
- 46. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90:2423–2431. [DOI] [PubMed] [Google Scholar]
- 47. Zumstein MA, Rumian A, Lesbats V, Schaer M, Boileau P. Increased vascularization during early healing after biologic augmentation in repair of chronic rotator cuff tears using autologous leukocyte- and platelet-rich fibrin (L-PRF): a prospective randomized controlled pilot trial. J Shoulder Elbow Surg. 2014;23:3–12. [DOI] [PubMed] [Google Scholar]