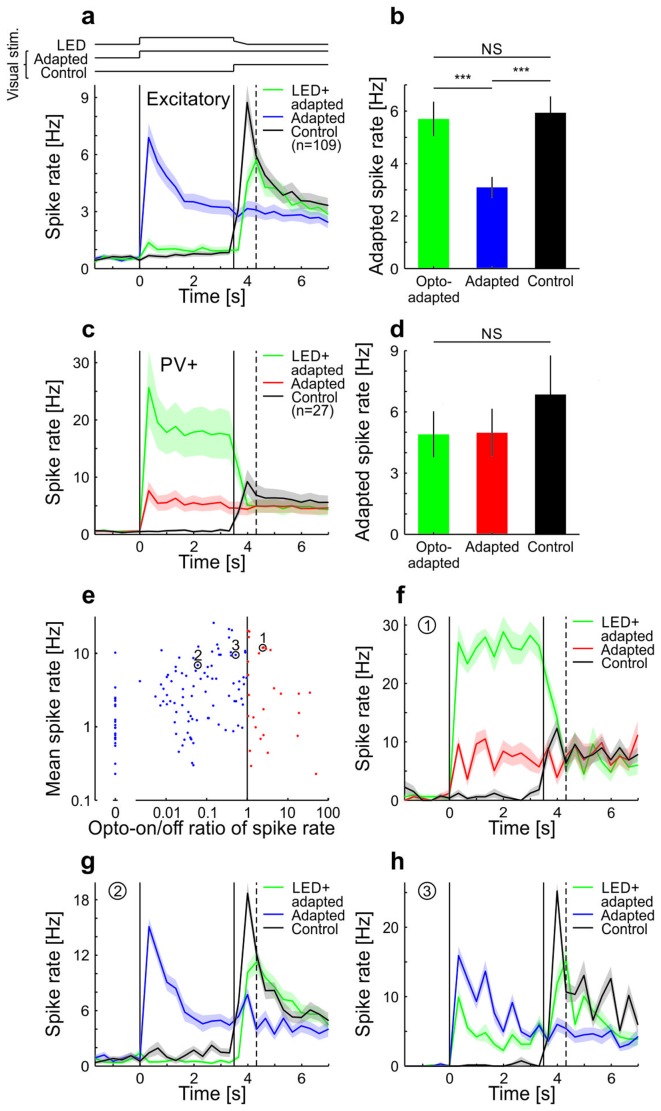
Under anesthesia, PV+ neurons adapted less than putative excitatory cells, consistent with the idea that they might inhibit other neurons to produce contrast adaptation (
Keller and Martin, 2015). Moreover, iso- and cross-orientation adaptation had similar effects on the population (see also
Stroud et al., 2012). In mouse V1, PV+ neurons are much less selective to a grating of different orientations than excitatory neurons (
Atallah et al., 2012;
Hofer et al., 2011;
Kerlin et al., 2010). Therefore, an adaptation mechanism based on PV+ neurons would result in adaptation that is only weakly dependent on the orientation of the stimulus. Indeed, adapting putative excitatory cells to their preferred or null (orthogonal to preferred) orientation results in a significant, but small difference in adapted firing rates. Moreover, no difference was found when adapting PV+ cells with either iso- or cross-orientation under anesthesia (with respect to test orientation). (
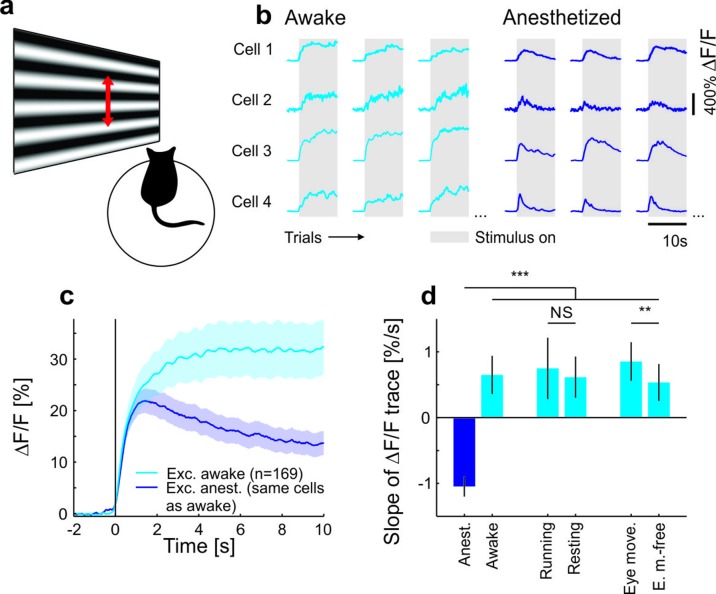
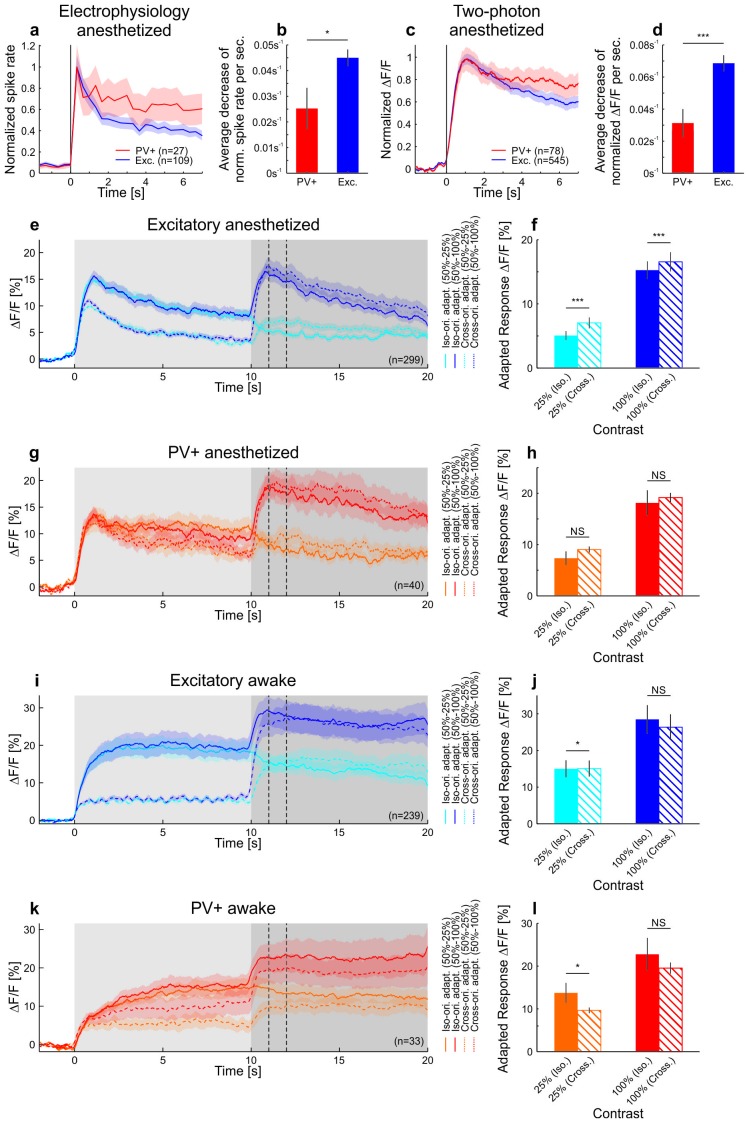
a) Neural activity was recorded with multi-tetrode arrays in anesthetized mice (same cells as in
Figure 1—figure supplement 1a,b). Averaged and normalized responses of tuned putative excitatory and PV+ cells to a moving square-wave grating at 100% contrast presented for 7 s in anesthetized mice. Curves plotted as mean ± SEM (shading). (
b) Adaptation is quantified as the mean decrease in the normalized spike rate per second during the stimulus presentation (tuned putative excitatory: 109 cells; PV+: 27 cells; p=0.010; Wilcoxon rank-sum). Bars plotted as mean ± SEM. (
c) The neural activity of tuned putative excitatory compared to PV+ cells recorded with two-photon calcium imaging. Averaged and normalized calcium responses to a moving sinusoidal grating at 50% contrast presented for 7 s in anesthetized mice. Curves plotted as mean ± SEM (shading). (
d) Adaptation is quantified as the mean decrease in normalized ∆F/F per second during the stimulus presentation (tuned putative excitatory: 545 cells; PV+: 78 cells; p=3.7×10
−4; Wilcoxon rank-sum). The initial rise was excluded in the estimation of the slope (see Materials and methods). (
e) Averaged responses of tuned putative excitatory cells in anesthetized mice to a moving sinusoidal grating at 50% contrast presented for 10 s followed by 10 s of a second stimulus of increased (100% contrast; dark traces) or decreased contrast (25% contrast; light traces). The first stimulus was either presented at the optimal (solid traces) or the orthogonal orientation of the neurons (dotted traces). The second stimulus was always presented at the optimal orientation. The two vertical dotted lines indicate the time window which was used to compare iso- and cross-orientation adaptation in
f. ∆F/F traces plotted as mean ± SEM (shading). (
f) Iso-orientation compared to cross-orientation adaptation (50% contrast) of tuned putative excitatory cells measured at 25% or 100% contrast. This is quantified as average ∆F/F after adaptation (seconds 11–12; indicated by the vertical dotted lines in
e; 299 cells; 25%: p<10
−10; 100%: p<10
−4; Wilcoxon signed-rank). (
g) Same as
e but for PV+ cells. (
h) Same as
f but for PV+ cells (40 cells; 25%: p=0.12; 100%: p=0.98; Wilcoxon signed-rank). (
i) Same as
e but in awake mice. Note that the absence of adaptation to the moving sinusoidal grating at 50% contrast (0–10 s) cannot be explained by a response ceiling since the cells increase their responses when increasing the contrast to 100% (at 10 s). (
j) Same as
f but in awake mice (239 cells; 25%: p=0.0082; 100%: p=0.14; Wilcoxon signed-rank). (
k) Same as
i but for PV+ cells. (
l) Same as
j but for PV+ cells (33 cells; 25%: p=0.0064; 100%: p=0.20; Wilcoxon signed-rank). Bars plotted as mean ± SEM. NS, not significant; *p<0.05; ***p<0.0005.