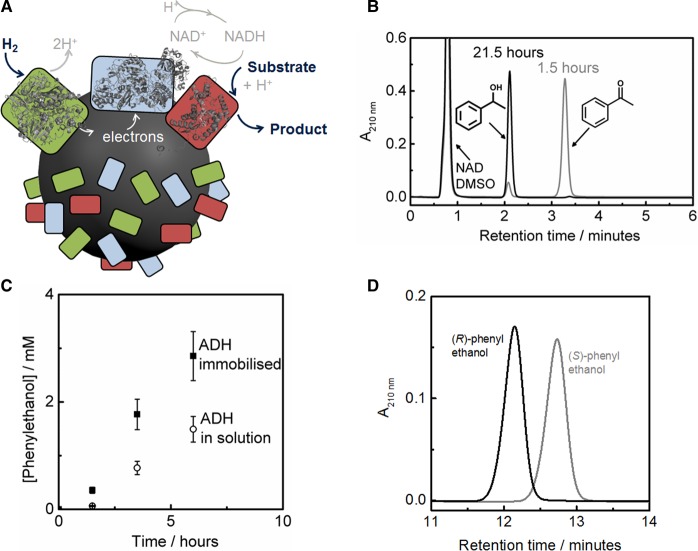
Figure 11. Enzyme-modified particles for highly selective H2-driven ketone reduction at 1 bar H2.
(A) Schematic representation of the particle system. (B) HPLC traces after 1.5 and 21 h of H2-driven ketone reduction. (C) The generation of phenylethanol using ADH co-immobilised on particles or in solution. Panels (B) and (C) are reproduced from ref. [45] by Reeve et al. (D) HPLC traces showing generation of (R)- or (S)-1-phenylethanol with >99% ee when co-immobilising different alcohol dehydrogenases; black = (R)-selective ADH 101 and grey = (S)-selective ADH 105, both provided by Johnson Matthey Catalysis and Chiral Technologies. All experiments made use of E. coli Hyd2 [18] and the NAD+-reducing moiety was HoxHI64AYFU of R. eutropha soluble hydrogenase, with the hydrogenase portion inactivated by an I64A exchange in the HoxH subunit (prepared according to established protocols) [46] immobilised on carbon black (BP2000). Reaction conditions: 10 mM acetophenone, 1 mM NAD+, Tris–HCl buffer (pH 8), 25°C, 2% DMSO.