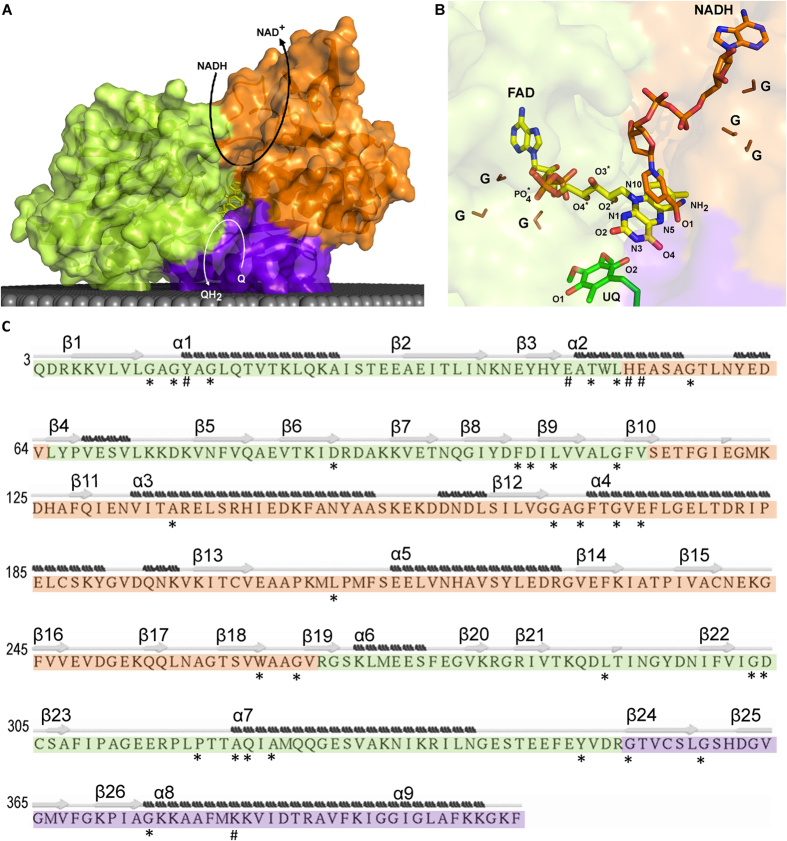
Figure 1. NDH-2 and substrates.
NDH-2 is composed of three structural domains: first dinucleotide binding domain, or FAD binding domain (green); second dinucleotide binding domain or NADH binding domain (orange); and membrane interacting domain, including two amphipathic helices at the C-terminal (purple). (A) Cartoon representation of the X-ray crystal structure of NDH-2 from S. aureus (PDB:4XDB7). The gray area represents the membrane and curved arrows schematize NADH:quinone oxidoreductase activity; (B) Cartoon representation of a zoomed view of the FAD region and co-crystallized ubiquinone and NADH of the NDH-2 from S. cerevisiae (PDB:4G734). The atoms of the FAD group are ordered and coloured in: blue – Nitrogen atom (N); red – Oxygen atom (O); orange – Phosphorus atom (P) and yellow – Carbon atom (C). The glycine residues composing the GxGxxG motif present each of dinucleotide binding domain are coloured in brown and indicated by “G”; (C) Sequence of NDH-2 from S. aureus7 indicating secondary structure elements. Secondary structure was predicted using STRIDE. β-sheets and α-helices are numbered from the N- to the C-terminal. Residues with at least 80% conservation (*) and with high covariance (#) are marked.