Figure 1.
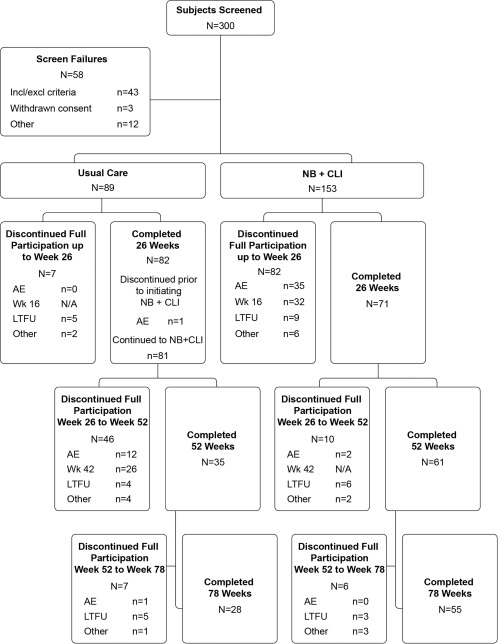
Study participants and group assignments. A total of 242 subjects were randomly assigned 1.75:1 to NB + CLI and usual care groups, all of whom were also treated and included in the intent‐to‐treat (ITT) analysis. AE, adverse event; LTFU, lost to follow‐up; other, protocol deviation or withdrawal of consent; N/A, not applicable; NB + CLI, naltrexone/bupropion and comprehensive lifestyle intervention; week 16, evaluation to continue treatment at week 16 visit; week 42, evaluation to continue treatment at week 42 visit.
