Figure 1.
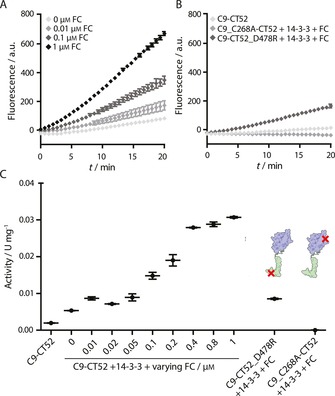
14‐3‐3‐scaffolded and small‐molecule‐induced caspase‐9 dimerisation probed by cleavage of a fluorogenic substrate. A) Fluorescent traces corresponding to cleavage of synthetic substrate Ac‐LEHD‐AFC (200 μm) in the presence of C9‐CT52 (0.1 μm), 14‐3‐3 (1 μm) and varying concentrations of FC (0, 0.01, 0.1 and 1 μm). B) Fluorescent traces corresponding to the following experiments: i) 0.1 μm C9‐CT52, ii) 0.1 μm C9_C268R‐CT52, 1 μm 14‐3‐3, and 1 μm FC, and iii) 0.1 μm C9‐CT52_D478R, 1 μm 14‐3‐3, and 1 μm FC. Ac‐LEHD‐AFC concentration is 200 μm in all experiments. C) Activity of caspase‐9 proteins (U mg−1) determined from the traces in (A) and (B). All experiments were conducted at 37 °C in assay buffer (20 mm Na2HPO4, 150 mm NaCl, 1 mm EDTA, 2 mm TCEP, pH 7.0). Each error bar represents the standard deviation in (A) and (B) and the standard error of the mean in (C) based on three independent measurements.
