Classification of evidence.
This is a single observational study without controls, providing Class IV evidence for the effectiveness of maraviroc in the treatment of progressive multifocal leukoencephalopathy–immune reconstitution inflammatory syndrome in MS.
Case report.
A 55-year-old Caucasian HIV-negative man diagnosed with relapsing-remitting MS in 2013 and an Expanded Disability Status Scale (EDSS) score of 2.0 had received 20 infusions of natalizumab over 21 months without a history of prior immunotherapy. John Cunningham–virus (JCV) antibodies were positive at the initiation of treatment (index: 0.4). Natalizumab was then discontinued because of an increase of anti-JCV antibody index (2.6) and switched to fingolimod following a 2-month washout period. Brain MRI performed 2 months before the initiation of fingolimod showed no signs of MS disease activity or progressive multifocal leukoencephalopathy (PML).
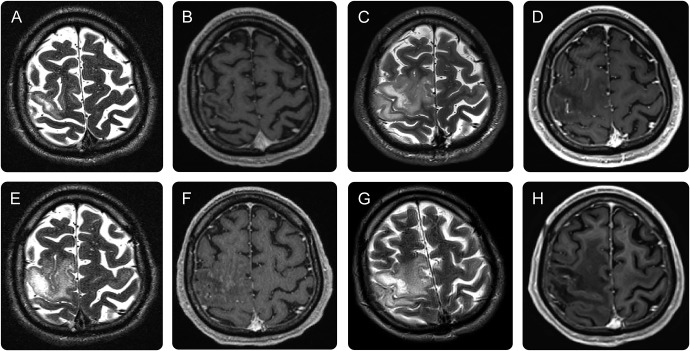
The patient was first admitted to our hospital 10 days after fingolimod initiation with progressive left-sided hemiparesis and an extensive T2 MRI lesion in the right central region without gadolinium (Gd)-enhancement suspicious for PML (figure, A and B). JCV DNA was detectable in the CSF (50 copies/mL) by PCR analysis.
Figure. Sequential MRIs of the brain.
(A, B) MRIs at admission show a lesion in the right central region without Gadolinium (Gd)-enhancement suspect of progressive multifocal leukoencephalopathy (PML). (C, D) MRIs after plasmapheresis show a markedly increased PML lesion size on T2 sequences but no Gd-enhancement. (E, F) Eight days after maraviroc initiation, MRIs reveal stable PML lesion size with inhomogenous spotty Gd-enhancement, consistent with moderate localized immune reconstitution inflammatory syndrome. (G, H) MRIs obtained 6 months after maraviroc initiation showed regression of PML lesion size in T2 without Gd-enhancement but a demarcated substance defect in T1 sequences.
The patient was diagnosed with natalizumab-associated PML, fingolimod was discontinued, and 4 cycles of plasmapheresis were conducted.
Clinically, the patient rapidly deteriorated to complete left-sided paralysis and developed focal seizures and a severe organic psychosyndrome, resulting in a 30% Karnofsky Index (KI).1 Correspondingly, MRI after plasmapheresis showed a markedly increased PML lesion size on T2 sequences but again no intraparenchymal Gd-enhancement (figure, C and D).
Oral maraviroc (300 mg twice daily) was initiated 6 days after admission. No glucocorticoids or any other immunomodulating therapy was given throughout the course of PML. Eight days after maraviroc initiation, MRI follow-up revealed stable PML lesion size with Gd-enhancement consistent with localized moderate immune reconstitution inflammatory syndrome (IRIS) (figure, E and F).
Over the following weeks, the patient continuously improved. PML lesion size regressed without Gd-enhancement (figure, G and H). After 25 weeks of maraviroc treatment, JCV DNA was no longer detectable in the CSF. Maraviroc was continued and well tolerated at a stable dose.
The patient survived both PML and IRIS with considerable persisting sequelae; after 10 months of treatment with maraviroc, the EDSS score was 3.0 without cognitive or psychiatric deficits. He was able to perform nearly all activities of daily living without support (KI 70%). Since there was neither clinical nor radiologic MS disease activity detectable during the follow-up period of 10 months, due to the lack of any recommendations (and even larger individual physicians' experience) on the use of a disease-modifying therapy (DMT) after survival of PML, and published reports of PML associated with other DMTs (such as fingolimod or dimethylfumarate), we have chosen not to start a new DMT yet.
Discussion.
PML is a rare but debilitating and often fatal infection of the CNS caused by JCV. It was initially described as a rare complication in immunocompromised patients. During the past decade, PML has been increasingly recognized as a risk for certain immunomodulatory treatments, especially in the context of natalizumab treatment in MS.
The IRIS can be a serious complication in patients with immunocompromised PML, usually occurring days to weeks after immunoreconstitution (PML-IRIS).1 While immunoreconstitution is vital for clearance of JC virus, development of IRIS poses a considerable threat of complications, disability, and death. Hence, management of PML-IRIS is critical. The common—though unproven—treatment of PML-IRIS with steroids may limit JC virus clearance.2 Recently, maraviroc was reported to be effective in both preventing and treating IRIS in natalizumab-associated PML.3 Maraviroc blocks C-C chemokine receptor type 5 (CCR5)-mediated inflammation and lymphocyte entry. It is approved for treatment of HIV infection and was also reported to reduce graft-vs-host disease in patients treated with allogenic bone marrow transplantation.4 CCR5 is involved in the recruitment of lymphocytes to the CNS, has direct implications in the pathophysiology of natalizumab-associated PML, and is strongly expressed on CD8-positive T cells in PML-IRIS brains.5,6
In general, mild clinical and MRI presentation as well as a low JC virus DNA load in the CSF are considered indicative for a favorable PML outcome.7 In fact, our patient rapidly developed severe PML. Subsequent maraviroc treatment was associated with rapid clinical improvement again. While there was some Gd-enhancement on MRI consistent with moderate IRIS, maraviroc seemed to effectively control IRIS, without the use of steroids and without IRIS causing complications or additional disability, and to presumably promote clearance of JC virus. PML-IRIS may stabilize and even improve after immune reconstitution. However, the rapid clinical and radiologic improvement of an early severe PML shortly after maraviroc initiation strongly suggests that the improvement was treatment related rather than mere potential natural recovery.
The optimal timing of initiation and duration of maraviroc treatment still has to be determined. However, it seems appropriate to start maraviroc as soon as the diagnosis of natalizumab-associated PML is established. Although efficacy determination will ultimately require a larger scale and appropriately controlled clinical trial, there are obvious limitations of a larger controlled clinical trial due to the orphan occurrence of PML. In conclusion, maraviroc currently seems to be the most promising option for prevention and treatment of PML-IRIS in MS, warranting further clinical confirmation.
Footnotes
Author contributions: Gabriel Bsteh: concept and design, patient recruitment, acquisition of data, and draft of manuscript. Michael Auer and Sarah Iglseder: acquisition of data and critical revision of manuscript for intellectual content. Lisa Walchhofer: acquisition of data, MRI, and critical revision of manuscript for intellectual content. Dietmar Langenscheidt, Stefan Koppi, Gabriele Schauer-Maurer, Guenther Stockhammer, and Thomas Berger: acquisition of data and critical revision of manuscript for intellectual content.
Study funding: No targeted funding.
Disclosure: G. Bsteh reports no disclosures. M. Auer received travel funding and/or speaker honoraria from Novartis and Biogen Idec. S. Iglseder, L.-M. Walchhofer, D. Langenscheidt, S. Koppi, G. Schauer-Maurer, G. Stockhammer, and T. Berger report no disclosures. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Tan IL, McArthur JC, Clifford DB, Major EO, Nath A. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology 2011;77:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 2009;72:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacomini PS, Rozenberg A, Metz I, et al. . Maraviroc and JC virus-associated immune reconstitution inflammatory syndrome. N Engl J Med 2014;370:486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reshef R, Luger SM, Hexner EO, et al. . Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med 2012;367:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwab N, Höhn KG, Schneider-Hohendorf T, et al. . Immunological and clinical consequences of treating a patient with natalizumab. Mult Scler 2012;18:335–344. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Blondel G, Bauer J, Uro-Coste E, et al. . Therapeutic use of CCR5 antagonists is supported by strong expression of CCR5 on CD8(+) T cells in progressive multifocal leukoencephalopathy-associated immune reconstitution inflammatory syndrome. Acta Neuropathol 2015;129:463–465. [DOI] [PubMed] [Google Scholar]
- 7.Dong-Si T, Gheuens S, Gangadharan A, et al. . Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol 2015;21:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]