Fig. 3. Concurrent study of VSV-G-BLAM-LV fusion/transduction with an immortalized human T cell line.
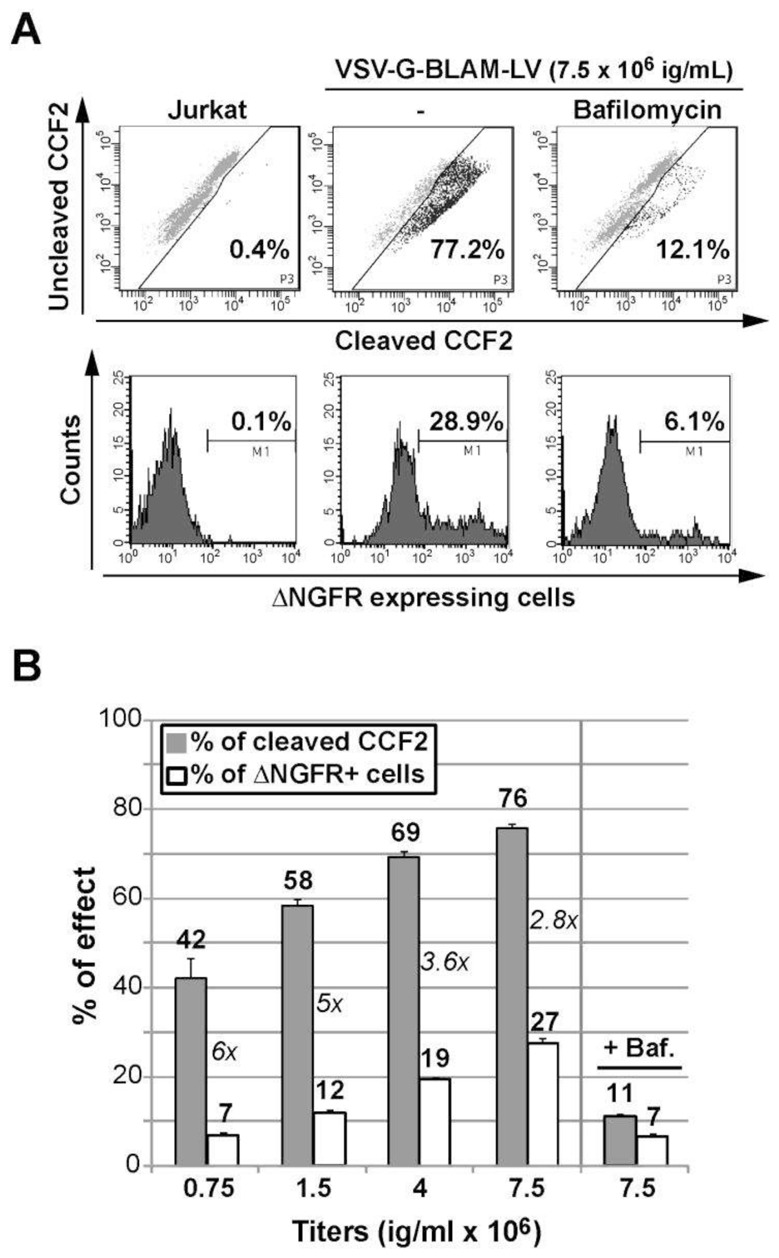
(A) Analysis of the fusion and the transduction of Jurkat T cells with VSV-G-BLAM-LV. The fusion-positive cell gate was established based on untransduced living cells loaded with the CCF2 substrate (upper left panel). In the presence of VSV-G-BLAM-LV, a control condition including the VSV-G fusion inhibitor Bafilomycin (150 nM) was used. The cell transduction efficiency with VSV-G-BLAM-LVs was monitored at day 5 by following NGFR expression at the cell surface (Lower panels). (B) Histograms are representing the fusion (gray) and the transduction (white) efficiency obtained for Jurkat T cells incubated with various infectious titers (ig/ml) of VSV-G-BLAM-LVs. A control condition including Bafilomycin (Baf.) was added for the highest dose of vector. Data are represented as the average values ± SD (n=3). The numbers in italic represent the ratio between fusion and transduction efficiency (F/T ratio) for each vector dose.
