Abstract
Rodents belong to the order Rodentia, which consists of three families in Borneo (i.e., Muridae, Sciuridae and Hystricidae). These include rats, mice, squirrels, and porcupines. They are widespread throughout the world and considered pests that harm humans and livestock. Some rodent species are natural reservoirs of hantaviruses (Family: Bunyaviridae) that can cause zoonotic diseases in humans. Although hantavirus seropositive human sera were reported in Peninsular Malaysia in the early 1980s, information on their infection in rodent species in Malaysia is still lacking. The rodent populations in residential and forested areas in Sarawak were sampled. A total of 108 individuals from 15 species of rodents were collected in residential (n = 44) and forested ( n = 64) areas. The species diversity of rodents in forested areas was significantly higher (H = 2.2342) compared to rodents in residential areas (H = 0.64715) (p < 0.001 of Zar-t test based on the Shannon index). Rattus rattus and Sundamys muelleri were present at high frequencies in both localities. An enzyme-linked immunosorbent assay (ELISA) showed that hantavirus-targeting antibodies were absent from 53 tested serum samples. This is the first report of hantavirus seroprevalence surveillance in rodent populations in Sarawak, East Malaysia. The results suggested that hantavirus was not circulating in the studied rodent populations in Sarawak, or it was otherwise at a low prevalence that is below the detection threshold. It is important to remain vigilant because of the zoonotic potential of this virus and its severe disease outcome. Further studies, such as molecular detection of viral genetic materials, are needed to fully assess the risk of hantavirus infection in rodents and humans in this region of Malaysia.
Keywords: ELISA, Hantavirus, Non-volant Small Mammals, Rodents, Seroprevalence
Abstrak
Rodent tergolong dalam kumpulan Rodentia yang terdiri daripada tiga Famili di Borneo (Contoh: Muridae, Sciuridae dan Hystricidae). Ini termasuk tikus, tupai dan juga landak. Golongan ini tersebar di serata dunia dan dianggap sebagai haiwan perosak yang mengancam manusia dan haiwan ternakan. Sebahagian spesies rodent adalah sumber pembawa jangkitan Hantavirus (Famili: Bunyaviridae) yang menyebabkan penyakit zoonotik kepada manusia. Walaupun serum manusia pernah dilaporkan seropositif pada Hantavirus di Semenanjung Malaysia pada awal tahun 1980, informasi tentang jangkitan pada spesies rodent di Malaysia masih kekurangan. Populasi rodent di kawasan perumahan dan kawasan hutan di Sarawak telah disampel. Sejumlah 108 individu daripada 15 spesies telah ditangkap di kawasan perumahan (n = 44) dan kawasan hutan (n = 64). Kepelbagaian rodent di kawasan hutan secara signifikan lebih tinggi (H = 2.2342) berbanding rodent di kawasan perumahan (H = 0.64715) (p < 0.001 ujian Zar-t berdasarkan indeks Shannon). Rattus rattus dan Sundamys muelleri amat kerap dijumpai di kedua dua lokaliti. Ujian imunosorben taut-enzim (ELISA) menunjukkan antibodi kursus kepada Hantavirus tidak dapat dikesan daripada 53 sampel serum yang telah diuji. Ini adalah laporan pertama tentang seroprevalensi hantavirus di Sarawak, Malaysia Timur. Hasil kajian menunjukkan bahawa penyebaran Hantavirus tidak berada dalam populasi tikus yang ditangkap di Sarawak, melainkan jika pengesanan di bawah prevalensi rendah iaitu di bawah tahap ambang. Kajian lebih lanjut seperti pengesanan molekul pada komponen viral genetik diperlukan untuk menilai sepenuhnya risiko jangkitan Hantavirus pada rodent dan juga manusia dalam kawasan kajian ini, di Malaysia.
Kata kunci: ELISA, Hantavirus, Mamalia Kecil Bukan Terbang, Rodents, Seroprevalensi
Rodents are gnawing-type, non-volant, small mammals with two pairs of continuously growing incisors. In Borneo, the order Rodentia forms 27.5% of the mammalian fauna that belong to 61 species in three families (i.e., Muridae, Sciuridae and Hystricidae) (Yasuma & Andau 1999; Payne & Francis 2007). Most rodents have many ecological variations, such as being nocturnal, diurnal and arboreal. Hantaviruses are single-stranded, enveloped, negative sense RNA viruses of the Bunyaviridae family (Elliott et al. 1991; Plyusnin et al. 1996). Haemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) are two types of rodent-borne zoonotic diseases in humans that are caused by hantaviruses (Bi et al. 2008). There are more than 80 known hantavirus reservoir host species. These include 51 rodent species, seven bat species from the order Chiroptera, and 20 shrew and mole species from the order Soricomorpha (de Oliveira et al. 2014). According to Kallio-Kokko et al. (2006), the most prevalent natural reservoir hosts for hantaviruses belong to four rodent genera (i.e., Apodemus, Rattus, Clethrionomys and Peromyscus). The transmission of hantaviruses to humans occurs through bites from infected animals or the inhalation of aerosolized contaminated excreta, such as urine and faeces (Calisher et al. 2006; Yusof et al. 2010; Lin et al. 2012). In West Malaysia, hantavirus seropositive rodents were previously recorded in several states, including Kelantan (n=4; 9.09%), Port Klang (n=14; 15.91%), and Penang and Perlis (n=17; 6.7%) (Lim et al. 1985; Lam et al. 2001). However, the seroprevalence and infection of hantavirus in rodent and human populations of East Malaysia are still largely unknown. There were also several reported cases of rodents infected with hantavirus in neighbouring countries [i.e., China (Lin et al. 2012; Wang et al. 2000), South Korea (Lim et al. 2012), Singapore (Wong et al. 1985,1988; Johannson et al. 2010), Indonesia (Plyusnina et al. 2009; Ibrahim et al. 2013), Thailand, Cambodia and Lao PDR (Nitatpattana et al. 2000; Reynes et al. 2003; Blasdell et al. 2011, 2016)], which suggested that there was a possibility for the circulation of hantavirus in the rodent populations in East Malaysia.
Rodent samplings were conducted at four residential and five forested areas in Sarawak (Figure 1) from October 2014 to April 2015. The residential areas selected in this study were comprised of villages in suburban and rural areas, while the forested areas were comprised of kerangas and mixed dipterocarp forests. The distance between the residential areas and the forested areas ranged from the nearest at 6.65 km (Sebayor Village and Samarahan forest) to 225.79 km (Ulu Serian Village and Samunsam Wildlife Sanctuary). Five sampling days were conducted at each sampling site, with a total of 50 cage traps deployed at residential areas and 100 cage traps set up at forested areas throughout the sampling periods. Cage traps were randomly distributed at a distance of approximately 10 m apart. The bait, including banana, pineapple and dried salted fish, was individually placed inside each cage trap. The success rates of trapping rodents in residential areas were between 0.020 and 0.080, while at forested areas, the success rates were between 0.008 and 0.054. The morphometric measurements of rodents, such as head and body length (HB), weight, tail length (TL), ear length (E), head length (HL), hind foot length (HF) and sex, were recorded following the guidelines provided by McKenna et al. (1997), Payne and Francis (2007) and Francis (2008) for species identification. Less than 400 μl of blood was collected from each euthanized rodent using a syringe with a 22-G needle by the cardiac puncture technique, following the protocols by Herbreteau et al. (2011). The blood sample was immediately spun at 4,000 rpm for five minutes to obtain clear serum. The serum samples were temporarily stored in an icebox at field sites and immediately transferred to a −20°C freezer in the laboratory.
Figure 1.
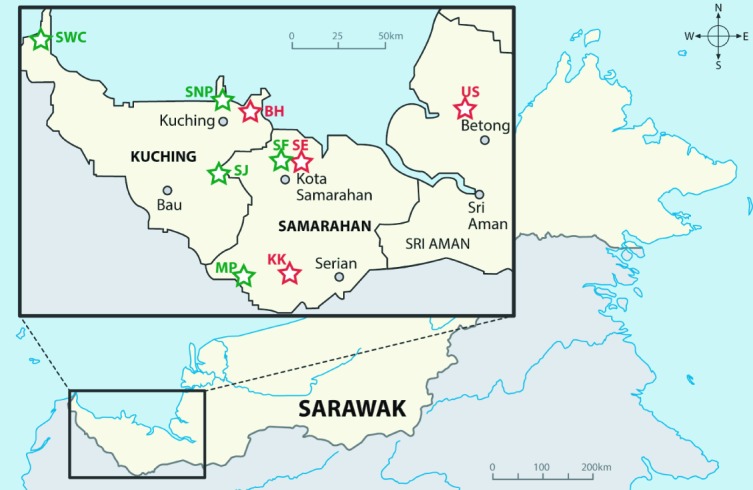
Sampling locations in Sarawak, East Malaysia, where the rodents were captured, and serum samples were screened for hantavirus-targeting antibodies.
Notes: Red asterisks indicate the residential sampling sites [KK, Krusen Kranji Village, Serian (1°5′15″N 110°30′40″E); SE, Sebayor Village, Kota Samarahan (1°27′34″N 110°29′56″E); US, Serian Ulu Village, Betong (1°50′0″N 111°40′0″E); and BH, Bako Hulu Village, Kuching (1°39′45″N 110°25′56″E)]. Green asterisks indicate forested sampling sites [SJ, Sama Jaya Nature Reserve (1°31′15″N 110°23′25″E); SNP, Santubong National Park (1°44′40″N 110°19′17″E); SWC, Samunsam Wildlife Sanctuary (1°57′11″N 109° 38′44″E); SF, Samarahan forest (1°28′33″N 110°26′11″E); and MP, Mount Penrissen (1°7′1″N 110°12′56″E)].
The screening of antibodies against hantaviruses from the rodent serum samples was performed in duplicate using the XpressBio Mouse Hanta Virus ELISA Kit (IM-100999; Express Biotech International, Thurmont, Maryland, USA), following the manufacturer’s protocol. The ELISA plate was pre-coated with negative and positive viral antigens, which were the recombinant hantavirus nucleoprotein of the Hantaan strain sharing high amino acid sequences homology with the existing Murinae-associated hantavirus strains. However, this test may miss the hantavirus of novel lineages.
A total of 108 rodent individuals belonging to 15 species were captured in the residential (n=44) and forested (n=6465) areas (Table 1): Callosciurus notatus, C. prevostii, Dremomys everetti, Leopoldamys sabanus, Maxomys baedon, M. ochraceiventer, M. surifer, M. whiteheadi, Niviventer cremoriventer, N. rapit, Rattus exulans, R. rattus, R. tiomanicus, Sundamys muelleri and Sundasciurus lowii. Only three rodent species (i.e., R. rattus, R. tiomanicus, and S. muelleri) were found in residential areas. Among these, R. rattus was the most commonly captured rodent from all residential areas. The presence of the invasive R. rattus at the Sama Jaya Nature Reserve (Sama Jaya NR) and Samarahan forest was not surprising, as these two forested areas were both located in the vicinity of the human settlements with urban landscapes. S. muelleri, a common rodent species in forested areas, was also commonly captured in the residential areas in this study, and it was possibly a commensal species to R. rattus. Urbanization has provided an alternative path for opportunistic rodent species such as S. muelleri to exploit urban or sub-urban habitats, whereas this species is usually found in forested areas (Wells et al. 2014). This study also captured four individuals of R. tiomanicus near a paddy field at Ulu Serian Village, Betong, Sarawak. The rodent species diversity was compared between the residential and forested areas based on the Shannon index calculated using PAleontological STatistic (PAST) version 3.10. As expected, the species diversity of rodents in forested areas (H = 2.23422.250) was significantly higher than in residential areas (H = 0.64715) [p<0.001, Zar-t test]. A total of 12 out of 15 rodent species captured were only recorded in forested areas. Among these, Sama Jaya NR had the most captured rodent individuals (n = 2627) compared to the other localities. Moreover, Sama Jaya NR is an urban park within kerangas forest reserve that provides both ecological and recreational services for researchers and local people. Ten individuals of rodents captured were R. rattus, a well-documented urban pest in Malaysia (Liat, 2015). The trapping success of rodents was lower at four other forested localities (Mount Penrissen, Samunsam Wildlife Sanctuary, Samarahan forest and Santubong National Park), where the majority vegetation consisted of mixed dipterocarp forests.
Table 1.
Rodent species captured in both residential and forested areas, Sarawak.
| Species name (Common name) | Total number of rodent individuals captured (number of sera collected for ELISA test) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Residential areasa | Forested areasb | Total | ||||||||
|
| ||||||||||
| KK | SE | US | BH | SJ | SNP | SWC | SF | MP | ||
| Sciuridae | ||||||||||
| Callosciurus notatus (Plantain squirrel) | 0 | 0 | 0 | 0 | 8(5) | 1(1) | 0 | 0 | 0 | 9(6) |
| Callosciurus prevostii (Prevost’s squirrel) | 0 | 0 | 0 | 0 | 1(0) | 0 | 0 | 0 | 0 | 1(0) |
| Dremomys everetti (Bomean mountain ground squirrel) | 0 | 0 | 0 | 0 | 0 | 0 | 1(0) | 0 | 3(0) | 4(0) |
| Sundasciurus lowii (Low’s squirrel) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1(1) | 1(1) |
|
| ||||||||||
| Muridae | ||||||||||
| Leopoldamys sabanus (Long-tailed giant rat) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6(1) | 6(1) |
| Maxomys baeodon (Small spiny rat) | 0 | 0 | 0 | 0 | 0 | 1(0) | 0 | 0 | 0 | 1(0) |
| Maxomys ochraceiventer (Chestnut-bellied spiny rat) | 0 | 0 | 0 | 0 | 0 | 1(0) | 0 | 0 | 0 | 1(0) |
| Maxomys surifer (Red spiny rat) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2(0) | 2(0) |
| Maxomys whiteheadi (Whitehead’s rat) | 0 | 0 | 0 | 0 | 0 | 0 | 4(2) | 1(0) | 1(0) | 6(2) |
| Niviventer cremoriventer (Dark-tailed tree rat) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3(0) | 3(0) |
|
| ||||||||||
| Niviventer rapit (Long-tailed mountain rat) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1(0) | 1(0) |
| Rattus exulans (Polynesian rat) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2(1) | 1(1) | 3(2) |
| Rattus rattus (House rat) | 5(3) | 18(8) | 2(2) | 10(8) | 10(4) | 0 | 0 | 4(3) | 0 | 49(28) |
| Rattus tiomanicus (Malaysian field rat) | 0 | 0 | 4(3) | 0 | 0 | 0 | 0 | 0 | 0 | 4(3) |
| Sundamys muelleri (Muller’s rat) | 0 | 2(2) | 2(1) | 1(1) | 8(1) | 1(1) | 0 | 4(4) | 0 | 18(10) |
|
| ||||||||||
| Total | 5(3) | 20(10) | 8(6) | 11(9) | 27(10) | 4(2) | 5(2) | 11(8) | 18(3) | 109(53) |
Notes:
Four residential areas in Sarawak (KK, Krusen Kranji Village, Serian; SE, Sebayor Village, Kota Samarahan; US, Serian Ulu Village, Betong; and BH, Bako Hulu Village, Kuching).
Five forested areas in Sarawak (SJ. Sama Jaya Nature Reserve; SNP, Santubong National Park; SWC, Samunsam Wildlife Sanctuary; SF, Samarahan forest; and MP, Mount Penrissen).
A total of 53 rodent serum samples from six genera and eight species were collected for the Hantavirus seroprevalence test (Table 1). All of the rodent serum samples tested negative for antibodies against hantaviruses using the ELISA test. This result suggested that the rodent populations at this region were not exposed to the virus before, and they did not have the antibody levels above the detection limit by the time blood sampling was conducted. The virus can remain in a rodent’s body after infection, and it is possibly detectable for up to 270 days, regardless of the IgM and IgG antibodies specific against hantavirus production (Lam et al. 2001). There was a probability that the rodents were exposed to hantavirus, but the antibody level declined after a few weeks of infection, which led to negative results. R. rattus was considered one of the reservoirs for hantavirus in Asian countries, such as China, Cambodia, Japan and Thailand (Nitatpattana et al. 2000; Wang et al. 2000; Reynes et al. 2003; Lokugamage et al. 2004). Hantavirus was previously reported in rodent populations in Peninsular Malaysia, Thailand and Singapore (Lim et al. 1985; Wong et al. 1985; Nitatpattana et al. 2000; Lam et al. 2001); therefore, the possibility of hantavirus circulation in rodent populations in Sarawak was suspected. Since this study involved a relatively small sample size (n = 53) of rodent blood specimens and small sampling areas relative to the whole region of Sarawak, it is expected that in the future, a more expansive surveillance with viral RNA detection and analysis would allow a thorough assessment of the public health risks of hantavirus infection in this region. Viral RNA detection by real-time polymerase chain reaction (PCR) has high sensitivity if the primers match the sequence of the virus genome present in the samples, and it is a powerful diagnostic tool to identify the prevalence of virus circulation at the time of sampling. Serological tests often could not distinguish between old and new infections. In conclusion, the screening of antibodies specific against hantaviruses from all rodent serum samples showed seronegative results.
ACKNOWLEDGEMENTS
We would like to thank Sarawak Forestry Department for granting the research permits to conduct the rodent samplings (permit no. NCCD.907.4.4(Jld.11)-4 and park permit no. 537/2014). We gratefully thank the staff from Faculty of Resource Science and Technology and Department of Zoology for their help in this project. This project was partially supported by the Malaysia Ministry of Education under Fundamental Research Grant Scheme (FRGS/963/2013(04)) and Niche Research Grant Scheme (NRGS/1088/2013(02)).
REFERENCES
- Bi Z, Formenty PBH, Roth CE. Hantavirus infection: A review and global update. The Journal of Infection in Developing Countries. 2008;2(1):3–23. doi: 10.3855/jidc.317. https://doi.org/10.3855/jidc.317. [DOI] [PubMed] [Google Scholar]
- Blasdell K, Cosson JF, Chaval Y, Herbreteau V, Douangboupha B, Jittapalapong S, Lundqvist A, Hugot JP, Morand S, Buchy P. Rodent-borne hantaviruses in Cambodia, Lao PDR, and Thailand. EcoHealth. 2011;8(4):432–443. doi: 10.1007/s10393-011-0725-7. https://doi.org/10.1007/s10393-011-0725-7. [DOI] [PubMed] [Google Scholar]
- Blasdell K, Morand S, Henttonen H, Tran A, Buchy P. Hantavirus seropositivity in rodents in relation to habitat heterogeneity in human-shaped landscapes of Southeast Asia. Spatial and Spatio-temporal Epidemiology. 2016;17:27–35. doi: 10.1016/j.sste.2016.04.002. https://doi.org/10.1016/j.sste.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: Important reservoir hosts of emerging viruses. Clinical Microbiology Review. 2006;19(3):531–545. doi: 10.1128/CMR.00017-06. https://doi.org/10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira RC, Guterres A, Fernandes J, D’Andrea PS, Bonvicino CR, de Lemos ERS. Hantavirus reservoirs: Current status with an emphasis on data from Brazil. Viruses. 2014;6(5):1929–1973. doi: 10.3390/v6051929. https://doi.org/10.3390/v6051929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott RM, Schmaljohn CS, Collett MS. Bunyaviridae genome structure and gene expression. Current Topics in Microbiology and Immunology. 1991;169:91–141. doi: 10.1007/978-3-642-76018-1_4. https://doi.org/10.1007/978-3-642-76018-1_4. [DOI] [PubMed] [Google Scholar]
- Francis CM. A field guide to the mammals of South-East Asia. 1st Ed. London, United Kingdom: New Holland Publisher; 2008. [Google Scholar]
- Herbreteau V, Jittapalapong S, Rerkamnuaychoke W, Chaval Y, Cosson JF, Morand S, editors. Protocols for field and laboratory rodent studies. Bangkok: Kasetsart University Press; 2011. [Google Scholar]
- Ibrahim IN, Shimizu K, Yoshimatsu K, Yunianto A, Salwati E, Yasuda SP, Koma T, Endo R, Arikawa J. Epidemiology of hantavirus infection in Thousand Islands Regency of Jakarta, Indonesia. Journal of Veterinary Medical Science. 2013;75(8):1003–1008. doi: 10.1292/jvms.12-0442. https://doi.org/10.1292/jvms.12-0442. [DOI] [PubMed] [Google Scholar]
- Johansson P, Yap G, Low HT, Siew CC, Kek R, Ng LC, Bucht G. Molecular characterization of two hantavirus strains from different rattus species in Singapore. Virology Journal. 2010;7(1):15–24. doi: 10.1186/1743-422X-7-15. https://doi.org/10.1186/1743-422X-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio-Kokko H, Laakkonen J, Rizzoli A, Tagliapietra V, Cattadori I, Perkins SE, Henttonen H. Hantavirus and Arenavirus antibody prevalence in rodents and humans in Trentino, Northern Italy. Epidemiology and Infection. 2006;134(4):830–836. doi: 10.1017/S0950268805005431. https://doi.org/10.1017/S0950268805005431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Chua KB, Myshrall T, Devi S, Zainal D, Afifi SA, Nerome K, Chu YK, Lee HW. Serological evidence of hantavirus infections in Malaysia. The Southeast Asian Journal of Tropical Medicine and Public Health. 2001;32(4):809–813. [PubMed] [Google Scholar]
- Liat LB. The house rodents and house shrew in Malaysia and Southeast Asia. UTAR Agriculture Science Journal. 2015;1(2):43–50. [Google Scholar]
- Lim TW, Ambu S, Baek LJ, Ju YK, Lee HW, Ng CS. Investigation on the presence of Hantaan or/and related virus, etiologic agentof Hemorrhagic Fever with Renal Syndrome (HFRS) among rodents in the seaport of Penang and Perlis. Tropical Biomedicine. 1985;2:73–79. [Google Scholar]
- Lim MY, Ryou J, Kim SY, Shin E, Yoo YJ, Yun SM, Noh YT, Han MG, Ju YR. Seroprevalence of Hantaviruses in small wild mammals trapped in South Korea from 2005 to 2010. Journal of Vector Ecology. 2012;37(1):97–101. doi: 10.1111/j.1948-7134.2012.00205.x. https://doi.org/10.1111/j.1948-7134.2012.00205.x. [DOI] [PubMed] [Google Scholar]
- Lin XD, Guo WP, Wang W, Zou Y, Hao ZY, Zhou DJ, Dong X, Qu YG, Li MH, Tian HF, Wen JF, Pluyusnin A, Xu J, Zhang YZ. Migration of Norway Rats resulted in the worldwide distribution of Seoul Hantavirus today. Journal of Virology. 2012;86(2):972–981. doi: 10.1128/JVI.00725-11. https://doi.org/10.1128/JVI.00725-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokugamage N, Kariwa H, Lokugamage K, Iwasa MA, Hagiya T, Yoshii K, Tachi A, Ando S, Fukushima H, Tsuchiya K, Iwasaki T, Araki K, Yoshimatsu K, Arikawa J, Mizutani T, Osawa K, Sato H, Takashima I. Epizootiological and epidemiological study of hantavirus infection in Japan. Microbiology and Immunology. 2004;48(11):843–851. doi: 10.1111/j.1348-0421.2004.tb03616.x. https://doi.org/10.1111/j.1348-0421.2004.tb03616.x. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Bell SK, Simpson GG. Classification of mammals: Above the species level. Two. New York: Columbia University Press; 1997. [Google Scholar]
- Nitatpattana N, Chauvancy G, Dardaine J, Poblap T, Jumronsawat K, Tangkanakul W, Poonsuksombat D, Yoksan S, Gonzalez JP. Serological study of hantavirus in the rodent population of Nakhon, Pathom and Nakhon Ratchasima Provinces Thailand. The Southeast Asian Journal of Tropical Medicine and Public Health. 2000;31(2):277–282. [PubMed] [Google Scholar]
- Payne J, Francis CM. A field guide to the mammals of Borneo. Kota Kinabalu: The Sabah Society; 2007. [Google Scholar]
- Plyusnin A, Vapalahti O, Vaheri A. Hantaviruses: Genome structure, expression and evolution. Journal of General Virology. 1996;77(11):2677–2687. doi: 10.1099/0022-1317-77-11-2677. https://doi.org/10.1099/0022-1317-77-11-2677. [DOI] [PubMed] [Google Scholar]
- Plyusnina A, Ibrahim IN, Plyusnin A. A newly recognized hantavirus in the Asian house rat (Rattus tanezumi) in Indonesia. Journal of General Virology. 2009;90(1):205–209. doi: 10.1099/vir.0.006155-0. https://doi.org/10.1099/vir.0.006155-0. [DOI] [PubMed] [Google Scholar]
- Reynes JM, Soares JL, Hüe T, Bouloy M, Sun S, Kruy SL, Marie FFS, Zeller H. Evidence of the presence of Seoul virus in Cambodia. Microbes and Infection. 2003;5(9):769–773. doi: 10.1016/s1286-4579(03)00149-7. https://doi.org/10.1016/S1286-4579(03)00149-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Yoshimatsu K, Ebihara H, Ogino M, Araki K, Kariwa H, Wang Z, Luo Z, Li D, Hang C, Arikawa J. Genetic diversity of hantaviruses isolated in China and characterization of novel hantaviruses isolated from Niviventer confucianus and Rattus rattus. Virology. 2000;278(2):332–345. doi: 10.1006/viro.2000.0630. https://doi.org/10.1006/viro.2000.0630. [DOI] [PubMed] [Google Scholar]
- Wells K, Lakim MB, O’Hara RB. Shifts from native to invasive small mammals across gradients from tropical forest to urban habitat in Borneo. Biodiversity and Conservation. 2014;23(9):2289–2303. https://doi.org/10.1007/s10531-014-0723-5. [Google Scholar]
- Wong TW, Chan YC, Lee HW. Haemorrhagic fever with renal syndrome in Singapore: A case report. The Southeast Asian Journal of Tropical Medicine and Public Health. 1985;16(4):525–527. [PubMed] [Google Scholar]
- Wong TW, Chan YC, Yap EH, Joo YG, Lee HW, Lee PW, Yanagihara R, Gajdusek DC, Gibbs CJ. Serological evidence of hantavirus infection in laboratory rats and personnel. International Journal of Epidemiology. 1988;17(4):887–890. doi: 10.1093/ije/17.4.887. https://doi.org/10.1093/ije/17.4.887. [DOI] [PubMed] [Google Scholar]
- Yasuma S, Andau M. Mammals of Sabah. Field guide & identification. Kota Kinabalu, Sabah: Sabah Wildlife Department.; 1999. [Google Scholar]
- Yusof FM, Md Ismail AIB, Ali NM. Modeling population harvesting of rodents for the control of Hantavirus infection. Sains Malaysiana. 2010;39(6):935–940. [Google Scholar]


