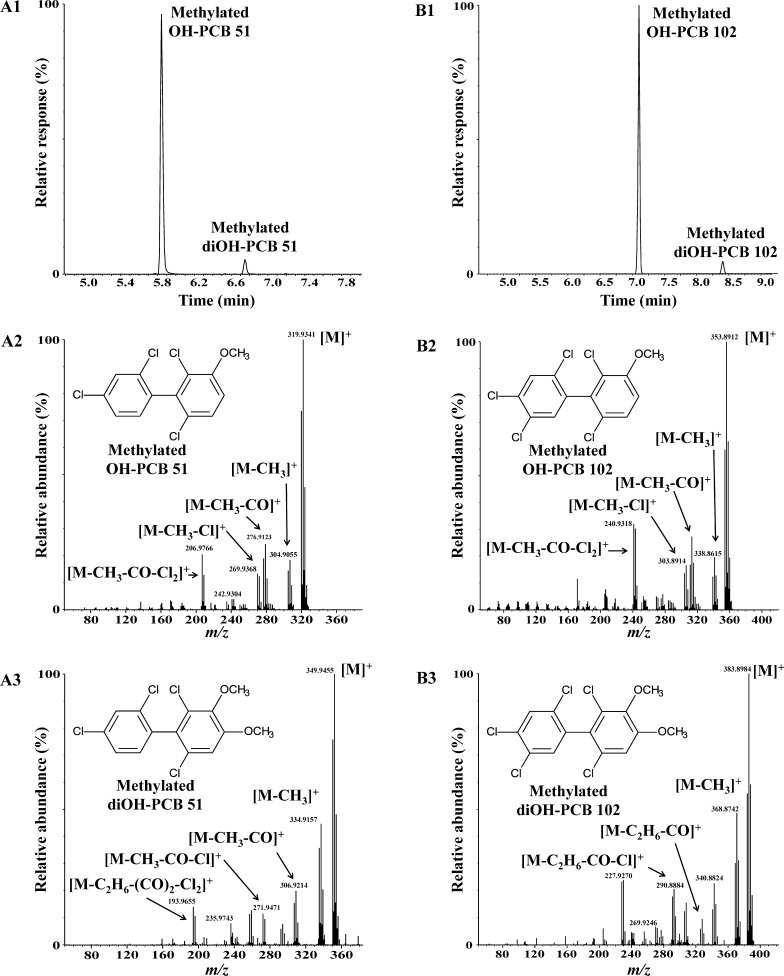
Figure 1.
Metabolism of prochiral PCB 51 or PCB 102 by rat liver microsomes results in the formation of one major monohydroxylated and a minor dihydroxylated metabolite (analyzed as the corresponding methylated derivatives). (A1) Gas chromatogram showing the presence of two metabolites, OH-PCB 51 and diOH-PCB 51, after incubation of PCB 51 with rat liver microsomes, and mass spectra of (A2) OH-PCB 51 and (A3) diOH-PCB 51 after derivatization with diazomethane. (B1) Chromatogram showing the presence of two metabolites, OH-PCB 102 and diOH-PCB 102, after incubation of PCB 102 with rat liver microsomes, and mass spectra of (B2) OH-PCB 102 and (B3) diOH-PCB 102 after derivatization with diazomethane. See text for additional details regarding the identification of these metabolites. Metabolism studies were performed for 2 h at 37 °C with rat liver microsomes from phenobarbital-pretreated male rats. Hexane/MTBE extracts of a representative incubation were analyzed after methylation of the OH-PCB metabolites with diazomethane by GC–MS as described in the Experimental Section.