Abstract
Previous studies of symbiotic associations between scleractinians corals and Symbiodinium have demonstrated that the consortium of symbionts can change in response to environmental conditions. However, less is known about symbiont shuffling during early coral development, particularly in brooding species. This study examined whether Symbiodinium consortia (1) varied in Porites astreoides on shallow (10m) and upper mesophotic (30m) reefs, (2) changed during coral development, and (3) influenced growth of juveniles in different environments. Symbiodinium ITS2 sequences were amplified using universal primers and analyzed using phylotype-specific primers designed for phylotypes A, B, and C. Adults from both depths were found to host only phylotype A, phylotypes A and B, or phylotypes A, B, and C and the frequency of the phylotype composition did not vary with depth. However, phylotype A was the dominant symbiont that was vertically transmitted to the planulae. The presence of phylotypes B and C was detected in the majority of juveniles when transplanted onto the shallow and upper mesophotic reefs whereas only phylotype A was detected in the majority of juveniles reared in outdoor aquaria. In addition, growth of juvenile P. astreoides harboring different combinations of Symbiodinium phylotypes did not vary when transplanted to different reef zones. However, juveniles reared in in situ reef environments grew faster than those reared in ex situ outdoor aquaria. These results show that Symbiodinium consortia change during development of P. astreoides and are influenced by environmental conditions.
Introduction
In recent decades, shallow coral reef communities have experienced large-scale declines driven by anthropogenic and environmental stressors [1–3]. Coral recovery is influenced by the frequency and magnitude of ongoing disturbances as well as the dynamics of population maintenance, recruitment, structure, and size [4]. Population maintenance and recruitment can occur through horizontal connectivity and vertical mixing, allowing for the increase in coral cover during both processes. These processes are shaped by patterns of dispersal and genetic connectivity, which vary with coral reproductive life histories. Broadcast spawning corals that undergo external fertilization, have been found to have high levels of genetic connectivity throughout the Caribbean and Western Atlantic, which may be attributed to high rates of cross-fertilization and a long pelagic dispersal period [5–7]. In contrast, brooding corals that undergo internal fertilization have lower levels of genetic connectivity throughout the Caribbean and Western Atlantic basin [8, 9], making local recruitment and juvenile acclimation crucial for population maintenance.
The brooding coral, P. astreoides is projected to be an important player on Caribbean and Western Atlantic reefs due to its increase in abundance, despite large-scale declines in coral cover and condition for other scleractinians species [10]. For example, in Curacao, relative abundance of juvenile P. astreoides (<4 cm in diameter) doubled between 1975–2005 [11]. In addition to an increase in relative abundance, P. astreoides has resilient qualities such as a genetic basis for thermal resilience [12, 13] and “weedy” life-history traits including its high levels of fecundity and settlement densities [10, 14, 15].
Though its increase in abundance and qualities of resilience positions P. astreoides to succeed on Caribbean reefs, little is known about P. astreoides early growth, symbioses during juvenile development, and mechanisms of acclimation to new environments. Knowledge of early growth and juvenile acclimation is essential in assessing population dynamics and recruitment success. To date, few studies have examined early growth and acclimation of juvenile brooding corals. When done so, these processes were monitored by collecting juveniles from the reef with subsequent experimentation [16–19] or by lab-rearing juveniles [20]. Early growth and symbioses has been more broadly examined in juveniles of broadcasting corals by inoculating aposymbiotic larvae with various strains of symbionts from the genus Symbiodinium and monitoring growth in lab or on the reef [21–26]. Having a deeper understanding of symbioses during juvenile growth and acclimation to differing environmental conditions is crucial to assessing P. astreoides population dynamics as it continues to succeed in the Caribbean-Western Atlantic region. Likewise, determining how these patterns differ in laboratory reared juveniles (ex situ) compared to those reared in the natural reef environment (in situ) is critical for interpretation of laboratory based studies on juvenile development and symbiotic relationships.
Important to these processes are photosynthetic symbionts from the genus Symbiodinium. These photosynthetic symbionts can provide the host coral with up to 100% of their energy requirements, making them crucial for coral growth and survival [27]. Currently there are nine genetically identified clades (A-I) of Symbiodinium that exhibit unique physiological traits and functional diversity [27, 28]. Harboring different combinations of Symbiodinium spp. and shuffling symbiont consortia can lead to enhanced coral fitness, growth, survival, and thermotolerance [22, 29–34]. Symbiont switching, or horizontal acquisition of Symbiodinium, leading to the accumulation of more thermotolerant symbionts (clade D Symbiodinium) has been found to be a strategy for environmental acclimation in adult corals during moderate warming events [14, 30, 34–36] and during recovery from bleaching events [33]. Alternatively, corals harboring clade C Symbiodinium often exhibit faster growth [31, 37] because clade C Symbiodinium translocates more carbon to the host relative to other Symbiodinium clades [21, 38]. However, these tradeoffs are compromised in the presence of thermal stress, where differences in growth of corals harboring different Symbiodinium clades is negligible [39]. Though it releases less carbon to the host in comparison to Symbiodinium clade C, members of clade A are thought to be highly productive [38], have a pigment profile which suggests potential for bleaching resistance [40], and possess pathways associated with increased photoprotection and survival in shallow water environments [41]. Additionally, adult P. astreoides harboring Symbiodinium type A4 maintain their symbiotic associations throughout bleaching events [42].
The evidence for symbiont flexibility in adult corals for acclimation to environmental change is widespread, however, this process is largely unexplored as a mechanism of juvenile acclimation. Previous studies have examined the flexible symbioses of Acropora spp. and Symbiodinium during horizontal symbiont acquisition by aposymbiotic larvae, revealing the ability of different types of Symbiodinium to confer increased coral growth and thermotolerance at early life stages [21–23, 26]. Similar to their adult counterparts, juveniles inoculated with clade C Symbiodinium exhibited faster growth while those harboring clade D Symbiodinium exhibited increased thermotolerance [22, 23, 26]. In broadcast-spawning corals releasing aposymbiotic larvae, such as Acropora spp., the process of symbiont-acquisition is highly flexible, where juveniles at 3.5 years did not mirror the Symbiodinium populations in their adult counterparts [22, 25]. In contrast, in brooding corals that release symbiotic planula, such as Stylophora pistillata, Symbiodinium consortia in nearby pelagic planulae and juveniles (<3 cm width) largely mirrored adult Symbiodinium consortia [16].
Coral life-history strategies are thought to play a role in patterns of symbiotic associations where broadcast-spawned, aposymbiotic larvae are provided an advantage to acquire Symbiodinium consortia appropriate for their local environment but failure to acquire Symbiodinium consortia will result in death [27]. Aposymbiotic larvae and subsequent juveniles are often highly flexible in their symbiotic associations during early development, with specificity occurring later in development [22, 43]. In contrast, brooding corals vertically transmit Symbiodinium where they are phagocytized during embryo endoderm formation and tissue layer differentiation [15, 44]. Brooded, symbiotic planulae do not risk death from failed symbiont acquisition though they risk receiving symbionts suitable for the local environment that are not necessarily well-suited for dispersal to new environments [27]. The process of symbiont shuffling during vertical transmission of Symbiodinium to brooded planulae and during early growth of resulting juveniles is poorly understood but have profound implications for juvenile acclimation.
The goal of this study was to examine variations in Symbiodinium consortia throughout various life history stages of the brooding coral, P. astreoides. Symbiotic associations with colonies of P. astreoides from different depths were examined to determine if the consortia of Symbiodinium associated with shallow (10m) and upper mesophotic (30m) colonies (1) differ between depths, (2) change during early development, and (3) influence growth of juveniles under varying environmental conditions. Here we present insight into conserved symbiotic associations throughout vertical transmission in brooding corals, and the influence of environment on Symbiodinium consortia associated with early juveniles and their growth.
Materials and methods
Study site
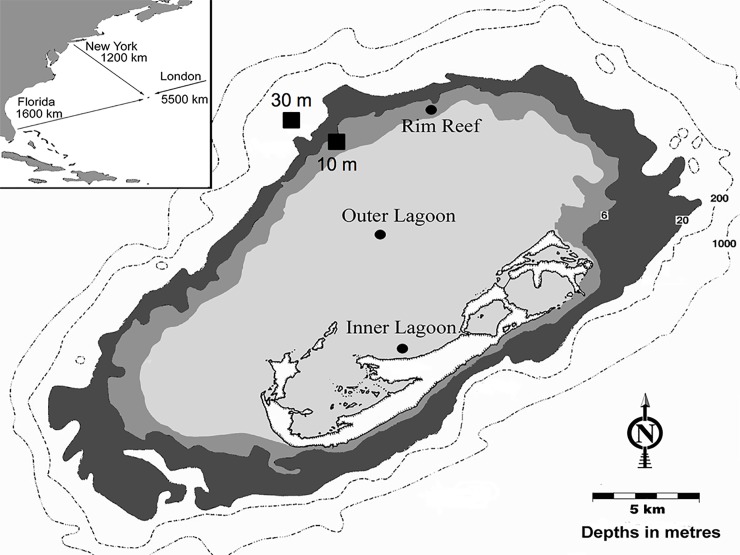
Collection of adult Porites astreoides and transplants of P. astreoides juveniles occurred at a shallow rim reef (10m, Hog Breaker) and an upper mesophotic (30m) reef in close horizontal proximity to each other (Fig 1; 10m: 32°45’77”N, 64°83’38”W; 30m: 32°48’80’N, 64°85’38”W). Adult P. astreoides colonies were collected from the aforementioned reefs on July 8 and August 5, 2015 following the guidelines of the Bermuda Institute of Ocean Sciences (BIOS) Collection and Experimental Ethics Policy’s “Limited Impact Research” policy (n = 10 per depth per month). Adult, planulae, and juvenile coral tissue samples were exported under CITES permits 15BM0010 and 15BM0014.
Fig 1. Map of shallow (10 m) and upper mesophotic (30 m) sampling sites in Bermuda.
Sites were used for adult Porites astreoides colony collection and juvenile transplantation.
Field and tissue collection methods
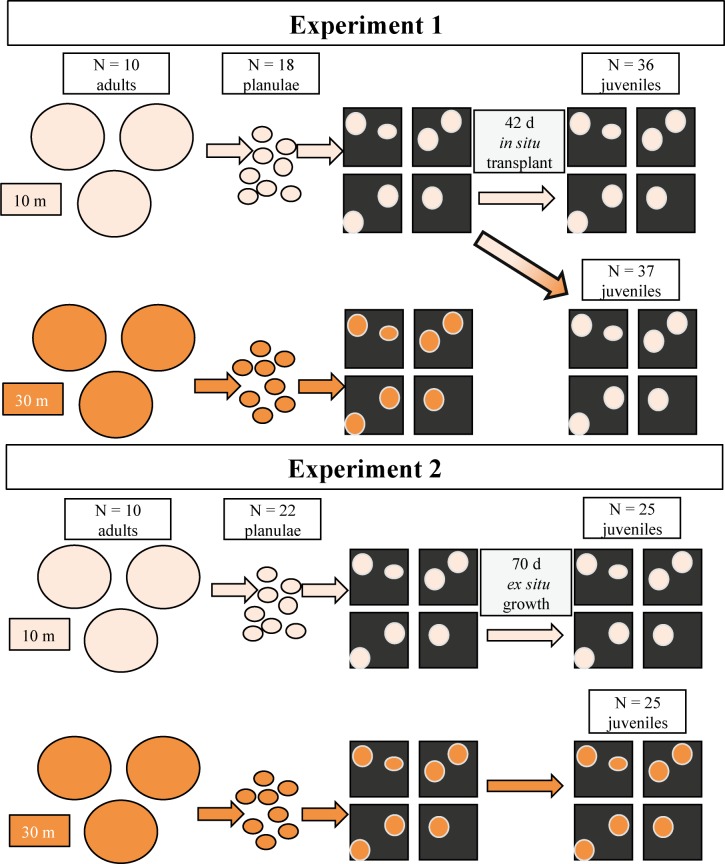
The field component of this study was composed of two experiments, the first conducted in July 2015 and the second in August 2015. In both Experiment 1 (July) and Experiment 2 (August), tissue was collected from adults, planulae, and juveniles to examine Symbiodinium consortia (Fig 2). However, in Experiment 1, juvenile spat from shallow adults were transplanted back to shallow and upper mesophotic reefs to examine in situ acclimation and juvenile growth rates. In Experiment 2, growth rates and Symbiodinium consortia were examined for juveniles produced from both shallow and upper mesophotic adults that were reared in ex situ laboratory conditions. See Fig 2 for a diagram of the experimental design.
Fig 2. Flow-through of experimental design for experiments 1 and 2.
In Experiment 1, Symbiodinium consortia were determined for shallow and upper mesophotic Porites astreoides adult colonies and planulae. Juveniles of shallow parental origin were reared in situ on the shallow and upper mesophotic reef and were examined for specific growth rates and Symbiodinium phylotype combinations. In Experiment 2, Symbiodinium consortia and specific growth rates were determined for shallow and upper mesophotic planulae and ex situ reared juveniles.
Experiment 1
Adult P. astreoides colonies were collected using a hammer and chisel, placed in individual plastic bags, and were transported to BIOS in coolers containing natural seawater. Colonies were then placed in individual containers in a shallow, ex situ, flow-through seawater system and planulation events were monitored following the methods outlined in [45]. On each day that planulation occurred (in both July and August), a subset of newly released planulae were preserved in 95% ethanol for molecular symbiont analyses (>6 planulae coral-1 day-1). The remainder of the newly released planulae were pooled by reef zone and settlement was assessed by placing 100 planulae into 20, 0.5 L plastic settlement chambers with 125 μm mesh tops (to allow for water flow) each containing two preconditioned terracotta tiles. After 1 week, the number and position of newly settled juveniles were scored under a dissecting microscope. Juveniles were photographed and surface area was determined using the “free-hand” tool in ImageJ™. The tiles with newly settled juvenile corals were then transplanted onto the same shallow (n = 17 tiles) and upper mesophotic (n = 22 tiles) reefs from which adult colonies were collected (Fig 1). Tiles were placed in a cage covered in egg crate and placed parallel to the ocean floor. When transplanted on July 29, 2015 the water temperature was 28.3°C at the shallow reef and 26.7°C at the upper mesophotic reef. When samples were retrieved on August 28, 2015 the water temperature was 28.3°C at the shallow reef and 25.5°C at the upper mesophotic reef. Following the transplant, surface area of individual juvenile P. astreoides was measured as described above and pre- and post-transplant surface areas were used to calculate specific growth rates (% growth d-1). Juvenile P. astreoides (36 per transplant depth) were removed from the tiles and preserved in 95% ethanol. Before adult colonies were returned to the reef, ~3 cm2 of coral tissue was removed using a waterpik, zooxanthellae were concentrated by centrifugation, resuspended in 1 mL of 95% ethanol and stored at -20°C [46].
Experiment 2
In August, 10 adult P. astreoides colonies were collected from the same shallow and upper mesophotic sites sampled in Experiment 1 (July) and planulae were collected in the manner described above. Symbiodinium were not isolated from these adult colonies, however, as outlined in the collection permit. Planulation was monitored and a subset of newly released planulae were preserved for molecular analyses with the remainder of planulae settled onto preconditioned terracotta tiles as in Experiment 1. After 1 week, newly settled juveniles from adults collected at both depths were photographed, sized using ImageJ™ and subsequently reared ex situ for 10 weeks in outdoor aquaria with flowing seawater at BIOS as described by [47]. Aquaria received continuous flowing seawater and were maintained under ambient light conditions and temperatures ranged from 24.0–29.4°C (S2 Fig). After 70 d, juveniles were photographed, sized, and specific growth rates were calculated as described above. Juvenile corals from both parental depths (n = 25 per depth) were then removed from the tiles and preserved in 95% ethanol for molecular analyses.
Molecular analyses
DNA was extracted from ethanol preserved planulae, juveniles, and adult coral tissue samples using a modified DE-1 protocol on a Kurabo QuickGene-810 AutoGene (Holliston, MA), where the tissue was ground with a pestle in a 1.5 mL tube after the addition of lysis buffer MDT, or a Qiagen DNeasy Plant Mini Kit following the manufacturer’s protocols (Valencia, CA). DNA concentration for each sample was spectrophotometrically quantified using a Thermo Scientific NanoDrop Lite spectrophotometer (Waltham, MA). For all samples, 1 μL of undiluted DNA was amplified in a 25 μL reaction with the following reagents: 17.75 μL dH2O, 2.5 μL New England Biolabs Taq Buffer (Ipswich, MA), 1 μL MgCl2, 1 μL 10 mM ITS-DINO (Table 1; [48]), 1 μL 10 mM ITS2rev2 (Table 1; [48]), 0.625 μL dNTP (10 mM each; New England Biolabs), and 0.125 μL Taq Polymerase (New England Biolabs). The concentration of DNA in each reaction ranged from 0.5–50.1 ng μL-1. The primers were designed to amplify the ITS2 region of all known Symbiodinium phylotypes [48]. DNA was amplified using a MJ Research Peltier Thermal Cycler (PTC-200; Ramsey, MN) with an initial denaturation at 95°C for 5 min, followed by 36 cycles of 94°C for 1 min, 57°C for 45 s, and 72°C for 30 s and a final extension at 72°C for 10 min. PCR products were visualized on a 2.0% Lonza NuSieve GTG Agarose (Walkersville, MD) gel in 0.5x Tris-Acetate-EDTA (TAE) buffer and run for 90 min at 80 V [49]. For samples that yielded two PCR products (upper band Porites astreoides 389 bp, lower band Symbiodinium sp. 313 bp), the smaller PCR product was gel-purified (Monarch Kit, New England Biolabs), re-amplified, and visualized as described above.
Table 1. PCR primers used in this study.
| Primer name | Region | Sequence 5’-3’ | Reference |
|---|---|---|---|
| ITS-DINO | ITS2 forward |
GTGAATTGCAGAACTCCGTG |
Pochon et al. 2001 |
| ITS2rev2 | ITS2 reverse |
CCTCCGCTTACTTATATGCTT |
Pochon et al. 2001 |
| Clade A ITS2 | ITS2 (clade specific) |
ATGGCACTGGCATGC |
Arif et al. 2014 |
| Clade B ITS2 | ITS2 (clade specific) |
ATTGCTGCTTCGCTTTCC |
Arif et al. 2014 |
| Clade C ITS2 | ITS2 (clade specific) |
TGCTTAACTTGCCCCAAC |
Arif et al. 2014 |
| Degenerate reverse primer | ITS2 (clade specific) |
TCWCYTGTCTGACTTCATGC |
Arif et al. 2014 |
Both strands of the PCR products were sequenced directly using single-pass Sanger sequencing services from Macrogen (Cambridge, MA) and assembled into individual contigs using CodonCode Aligner v3.5.6 (CodonCode Corporation, Centerville, MA). All sequences were compared with sequences in the non-redundant nucleotide collection at NCBI using BLASTN [50] and were identified as Symbiodinium type A4. Sequences were aligned using the ClustalW algorithm in MacVector v14.5.3 (MacVector, Inc, Apex, NC) and a consensus sequence was created, which was identical to Symbiodinium phylotype A4 (CCMP2456, LK934674.1) deposited in NCBI. A pairwise percent similarity matrix was generated (ignoring gaps) using Geneious v9.1.3 [51]. All sequences and sample information are publically available via Clark University’s Digital Commons (http://commons.clarku.edu/facultyworks/24/) and a representative sequence was deposited on Genbank (accession number KY273433).
ITS2 PCR products were used to screen for the presence/absence of phylotypes A, B, and C using 1 μL of degenerate reverse primer (10 mM) and 1 μL of forward primers (10 mM) designed to amplify phylotype A, B, or C (Table 1; [52]). PCR products representing each phylotype were visualized on a 2.0% Lonza NuSieve GTG Agarose gel in 1x Tris-borate-EDTA (TBE) buffer and run for 120 min at 80 V [49]. Samples were scored as positive for the individual phylotypes based on the presence of appropriately sized PCR product (phylotype A 100 bp, phylotype B 180 bp, and phylotype C 210 bp). For confirmation, PCR products were gel purified using a New England Biolabs Monarch kit, amplified, and sequenced directly using single-pass Sanger sequencing services from Macrogen (Cambridge, MA). Representative sequences for phylotypes B and C were deposited on Genbank (accession numbers KY273434-35, respectively).
Statistical analyses
Chi-squared analyses were used to test for differences in the frequency of occurrence of Symbiodinium phylotypes among adult, planulae, and juvenile P. astreoides. A two-by-c Tukey-type multiple comparisons of proportions was used to determine whether the proportion of corals harboring only Symbiodinium phylotype A or Symbiodinium phylotype A in combination with other phylotypes varied independently in different life stages [53]. A two-factor Analysis of Variance (ANOVA) was used to test for differences in the specific growth rates (% growth) of in situ transplanted juveniles using symbiont phylotype and transplant depth as factors. Growth rates (% growth d-1) did not meet assumptions of parametric statistics so a randomized two-factor ANOVA with 10,000 permutations was used [54]. A randomized one-factor ANOVA with 10,000 permutations [54] and pairwise posthoc comparison using Tukey and Kramer (Nemenyi) test with Tukey-Dist approximation was used to test for differences in the specific growth rates (% growth d-1) of juveniles reared in situ at different depths and ex situ of different parental depths. Statistical analyses were performed in R [55].
Results
DNA from adult corals, planulae, and juveniles were initially amplified using PCR primers that amplify the ITS2 region of all known Symbiodinium phylotypes [48]. All samples (n = 210) were identified as Symbiodinium type A4 and had an average percent similarity of 99.96% ± 0.02% (standard error) to the reference Symbiodinium type A4 sequence (CCMP2456, LK934674.1) deposited in NCBI. Percent similarity to the reference sequence ranged from range of 98 and 100%.
Symbiodinium consortia across depths
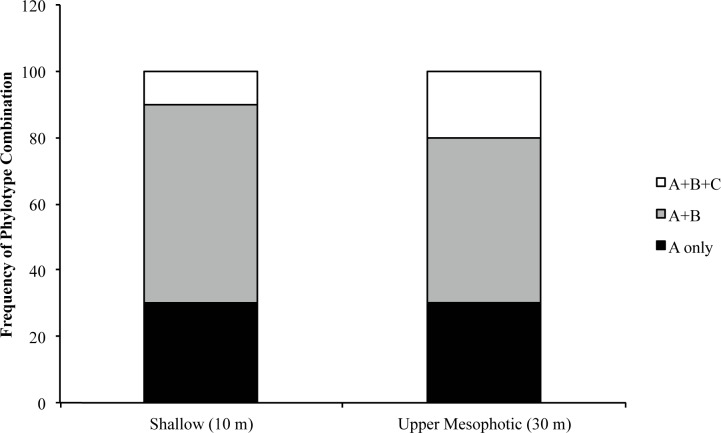
Although Symbiodinium phylotype A4 was the most abundant phylotype in P. astreoides, Symbiodinium phylotypes B and C were detected in nested PCR reactions using clade specific primers. Adults from both shallow (n = 10) and upper mesophotic (n = 10) reefs had three symbiont combinations: only phylotype A, phylotypes A and B, and phylotypes A, B, and C (Fig 3). The combination of Symbiodinium phylotypes A and B was detected at the highest frequency in adults from both shallow and upper mesophotic reefs and the distribution of phylotypes did not vary with depth (χ2 = 0.42, d.f. = 2, p = 0.81).
Fig 3. Symbiodinium phylotype composition of field-collected shallow and upper mesophotic adult Porites astreoides.
There was no significant difference in Symbiodinium phylotype frequency between depths.
Vertical transmission of Symbiodinium consortia
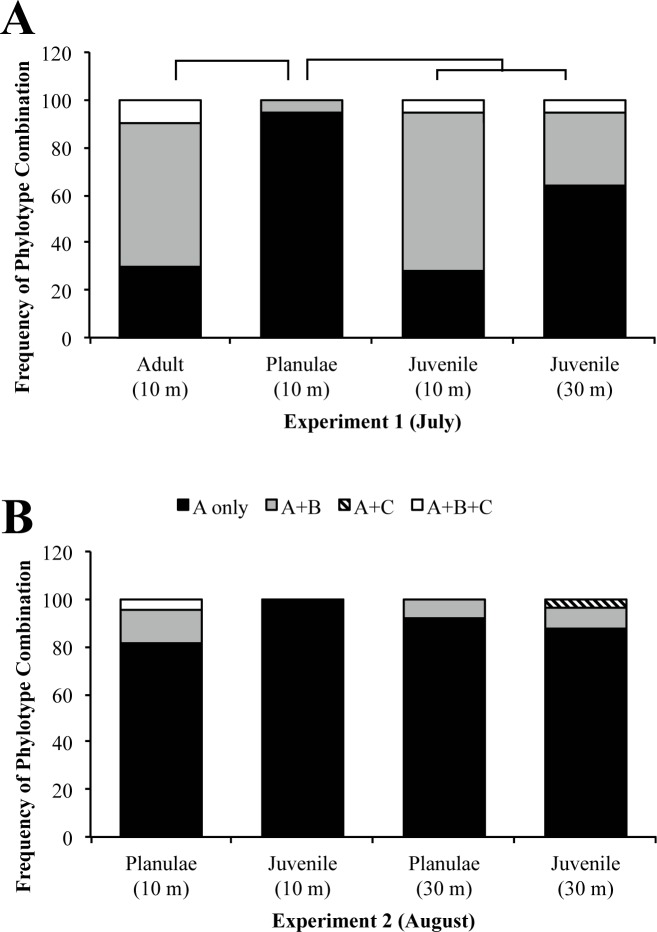
Symbiodinium phylotype frequencies varied significantly by developmental stage for shallow adult P. astreoides, the planulae they released, and resulting early juveniles (χ2 = 230.18, d.f. = 6, p = 7.02e-47). A Tukey-type multiple comparisons of proportions of corals harboring only Symbiodinium phylotype A versus Symbiodinium phylotype A in combination with other phylotypes was used to determine variation between the specific life stages (Table 2; [53]). Symbiodinium phylotype frequencies were significantly different in shallow adult P. astreoides and the planulae they released (Table 2; q = 5.06). Shallow (n = 10) adult colonies had three symbiont compositions: phylotype A only, phylotypes A and B, and phylotypes A, B, and C (Fig 4). This pattern was not observed in their planulae (n = 18) in which the majority of planulae contained only phylotype A and with the rest of the planulae containing both A and B phylotypes (Fig 4). Symbiodinium phylotype C was not detected in the planulae collected for analyses.
Table 2. Tukey-type multiple comparison of proportions of Symbiodinium phylotype frequencies in P. astreoides life stages.
| Comparison | p'B-p'A | SE | q | Critical q0.05,∞,4 |
|---|---|---|---|---|
| Adult vs. planulae | 39.59 | 7.83 | 5.06 | 3.63 |
| Planulae vs. juveniles transplanted to shallow reef | 41.73 | 5.78 | 7.22 | 3.63 |
| Planulae vs. juveniles transplanted to upper mesophotic reef | 21.03 | 5.78 | 3.64 | 3.63 |
| Shallow juveniles vs. upper mesophotic juveniles | 20.70 | 4.74 | 4.37 | 3.63 |
Fig 4.
Symbiodinium phylotype composition in various Porites astreoides life stages reared in situ (A) and ex situ (B). (A) Symbiodinium phylotype composition of shallow P. astreoides adults, their brooded planulae, and newly settled juveniles reared in situ on shallow (10 m) and upper mesophotic (30 m) reefs. Symbiodinium phylotype frequencies differed significantly between (1) adults and planulae, (2) planulae and juveniles transplanted to the shallow reef, (3) planulae and juveniles transplanted to the upper mesophotic reef, and (4) between juveniles transplanted to the different depths. There was no difference in Symbiodinium phylotype frequencies between adults and juveniles transplanted to different depths. (B) Symbiodinium phylotype composition of planulae and ex situ, reared juvenile P. astreoides. No difference was found in Symbiodinium phylotype frequency of (1) planulae or juveniles from different parental depths or (2) between planulae and juvenile.
Symbiodinium consortia in juvenile corals reared in situ vs. ex situ
After being transplanted to the shallow and upper mesophotic reef, Symbiodinium phylotypes A, B, and C were detected in the juvenile P. astreoides (Fig 4). The majority of juveniles transplanted to the shallow reef harbored phylotypes A and B whereas juveniles transplanted to the upper mesophotic reef harbored only phylotype A (Fig 4). Juveniles transplanted to the shallow reef and upper mesophotic reefs had significantly different Symbiodinium phylotype frequencies than the source population of shallow planulae (Fig 4; Table 2; q = 7.22; q = 3.64). Symbiodinium phylotype frequencies also varied significantly between the juveniles transplanted to different depths (Fig 4; Table 2; q = 4.37). In addition, juveniles transplanted to both depths had similar Symbiodinium phylotype frequencies as the shallow adult colonies (shallow, q = 0.30; upper mesophotic, q = 2.62).
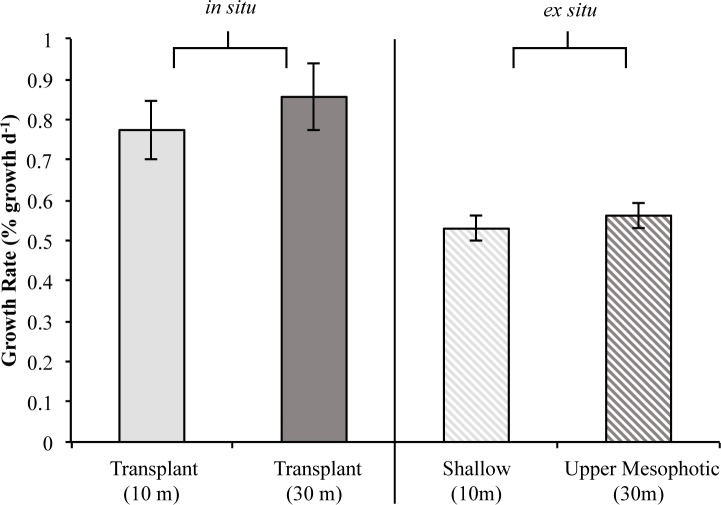
There was no significant difference in the average specific growth rates of juveniles transplanted onto the shallow and upper mesophotic reefs (t-test, p = 0.46). Juveniles transplanted to the shallow reef had an average growth rate of 0.77±0.07 (% growth d-1 ± standard error) whereas juveniles transplanted to the upper mesophotic reef had an average growth rate of 0.86±0.08 (Fig 5). Additionally, there was no difference in juvenile growth with the factors Symbiodinium phylotype composition (F = 0.022, p = 0.98) and transplant depth (F = 0.003, p = 0.96), or their interaction (F = 0.524, p = 0.58). Likewise, no difference was found in average percent mortality of juveniles transplanted to the shallow (40.49%±9.40%; average ± standard error) and upper mesophotic (35.37%±8.22%) reefs (t-test, p = 0.68).
Fig 5. Specific growth of in situ and ex situ reared Porites astreoides.
Specific growth of juvenile P. astreoides reared on shallow (10 m) and upper mesophotic (30 m) reefs (in situ) did not differ using transplant depth and Symbiodinium phylotype combinations as factors (left). Specific growth of shallow and upper mesophotic juvenile P. astreoides reared in outdoor aquaria (ex situ) also did not differ (right). Both treatments of in situ reared juveniles (left) had significantly higher growth rates than both treatments of ex situ reared juveniles (right). Shaded bars represent mean specific growth (% growth d-1) ± SE.
In Experiment 2 (August), there was no difference in Symbiodinium phylotype frequencies between shallow and upper mesophotic planulae (χ2 = 1.52, d.f. = 2, p = 0.47). The majority of shallow and upper mesophotic planulae harbored only phylotype A with the remaining harboring phylotypes A and B (Fig 4). All shallow juveniles harbored exclusively phylotype A; whereas the majority of upper mesophotic juveniles harbored only phylotype A with the remaining few harboring either phylotypes A and B, or phylotypes A and C (Fig 4). In addition, Symbiodinium phylotype frequencies varied but were not significantly different when comparing planulae and juveniles from either shallow (χ2 = 4.97, d.f. = 2, p = 0.083) or upper mesophotic parental origin (χ2 = 0.98, d.f. = 2, p = 0.61). There was no difference in Symbiodinium phylotype frequencies or growth rates between shallow and upper mesophotic juveniles raised in ex situ outdoor aquaria for 10 weeks (Fig 4; Fig 5; χ2 = 3.19, d.f. = 2, p = 0.20). Juveniles of shallow parental origin had an average growth rate of 0.53±0.03 (% growth d-1 ± standard error) and those of upper mesophotic parental origin had an average growth rate of 0.56±0.03 (Fig 5). In addition, there was no significant difference in average percent mortality of shallow (62.63%±6.33%; average ± standard error) and upper mesophotic (74.22%±5.40%) juveniles reared ex situ in outdoor aquaria (t-test, p = 0.17).
There was no significant difference in Symbiodinium phylotype frequency between shallow planulae from July and August, with the majority of samples only harboring phylotype A (χ2 = 1.65, d.f. = 2, p = 0.44; Fig 4). However, there was a difference in Symbiodinium phylotype frequency between juveniles reared on reefs compared with those reared ex situ in outdoor aquaria at BIOS. Juveniles reared on either shallow or upper mesophotic reefs had an increased presence of Symbiodinium phylotypes B and C compared with juveniles reared in outdoor aquaria. Symbiodinium phylotype frequencies varied significantly between juveniles of shallow parental origin reared on a shallow reef and juveniles of shallow parental origin grown ex situ shallow water conditions (χ2 = 31.47, d.f. = 2, p = 1.47e-7; Fig 4). There was a difference in Symbiodinium phylotype frequency between juveniles of shallow parental origin reared on an upper mesophotic reef and upper mesophotic juveniles reared in ex situ shallow water conditions, (χ2 = 5.51, d.f. = 3, p = 0.057).
There was a significant difference in the specific growth (% growth d-1) of juveniles reared in different environments (in situ, [shallow and upper mesophotic reefs] vs. ex situ, [outdoor aquaria]; % growth d-1) using experimental group as a factor (F = 10.1, d.f. = 3, p = 2.05e-6). Pairwise comparisons using Tukey & Kramer (Nemenyi) tests with Tukey-Dist approximations were used to compare specific growth of juvenile P. astreoides transplanted to shallow and upper mesophotic reefs (in situ) and shallow and upper mesophotic juveniles reared in outdoor aquaria (ex situ). Juveniles transplanted to either the shallow or upper mesophotic reef grew significantly faster than shallow juveniles (p = 0.003, p = 7.10e-6; respectively) and upper mesophotic juveniles (p = 0.011, p = 5.30e-5; respectively) reared in outdoor aquaria (Fig 5).
Discussion
Effects of depth on Symbiodinium consortia
Physiological diversity among Symbiodinium phylotypes supports coral distribution across a wide variety of environments ranging from those with high light levels [41, 56, 57] to those adapted to the low-light and temperatures associated with the lower mesophotic (60-100m; [58]). This study examined Symbiodinium consortia in Porites astreoides adults over a depth gradient where environmental factors such as temperature, nutrients, and light vary with depth. In Bermuda, seawater temperatures are warmer and more variable on shallow reefs compared with deeper reefs [59]. In addition, concentrations of nitrate and nitrite were found to be higher at shallow depths than deeper depths (10 and 45 m, respectively; [59]). Offshore of Bermuda, the light extinction coefficient is estimated to range between 0.025–0.55 m-1 during July and August, resulting in a gradual decrease in light between 10 and 30 m depth [60]. Despite these variations, there was no difference in the frequencies of Symbiodinium phylotypes in adult P. astreoides residing at 10 and 30 m (Fig 3). In contrast, variations in the distribution of Symbiodinium phylotypes associated with adult P. astreoides have been observed along depth gradients in several locations throughout the Caribbean [9, 30, 61, 62]. The lack of differentiation in Symbiodinium consortia in P. astreoides in our study may reflect less steep environmental gradients in Bermuda compared with regions in the Caribbean. Additionally, shallow and upper mesophotic P. astreoides in Bermuda have high levels of genetic connectivity and are thought to belong to a regionally isolated and locally sustained coral population which does not exhibit symbiont depth zonation [9].
We detected greater diversity in Symbiodinium than reported in other studies of P. astreoides in Bermuda [9, 40, 63]. The increased diversity in Symbiodinium observed here may be a result of differences in sampling locations. In this study, P. astreoides were collected from a northern rim reef, which has higher coral cover and diversity [64] relative sites selected in previous studies [9]. High coral density and diversity may lead to increased Symbiodinium diversity and availability in the water column [65], which in turn, may translate to the increased diversity of phylotypes observed in P. astreoides adults at these locations. Alternatively, the increased diversity observed in our study may reflect the nested PCR approach used to screen for specific Symbiodinium phylotypes. Recent studies have shown that amplification and direct sequencing or DGGE analyses of Symbiodinium ITS2 are unable to detect phylotypes that are in low abundance (e.g. less than 5–10% of the population; [35, 66, 67]). In this study, the first round of PCR used ITS2 primers that amplify all known Symbiodinium phylotypes and may have increased the abundance of phylotypes B and C amplicons to a level that was detected in the second round of PCR using clade-specific primers. Future studies that take advantage of quantitative PCR methods will be important to quantify the relative abundances of the different phylotypes.
Vertical transmission of Symbiodinium and variations in early development
Though the presence of phylotypes B and C in combination with phylotype A was found in the majority of adult corals, this pattern was not observed in planulae, of which all harbored phylotype A with the majority having only phylotype A (Fig 3). The decrease in diversity of Symbiodinium associated with P. astreoides planulae may be attributed to unique microenvironments and energetic requirements associated with brooding. P. astreoides eggs develop and planulae are brooded throughout the mesenteries, which may provide unique microenvironments within the corals [15, 68, 69]. The energy demands of brooded planulae are thought to be derived from maternal lipids and protein reserves rather than Symbiodinium, thus exogenous uptake or internal proliferation of non-dominant Symbiodinium may occur later in development after transitions in the metabolic interactions between the host and symbiont [70]. In the Red Sea, Byler et al. [16] found that the dominant Symbiodinium type in adult colonies of the brooding coral Stylophora pistillata to be vertically transmitted to planulae. This pattern occurred throughout the bathymetric distribution of S. pistillata in which Symbiodinium phylotypes varied with depth [16].
The increase in Symbiodinium phylotypes observed in reef-reared juveniles compared to their planulae suggests that horizontal Symbiodinium transmission during early development in brooding corals (Fig 4). When transplanted to the shallow reef, juveniles appeared to mirror shallow adult P. astreoides Symbiodinium phylotype combinations whereas juveniles transplanted to the upper mesophotic reef appeared to be in the process of mirroring mesophotic adult P. astreoides Symbiodinium consortia (Fig 3, Fig 4). It is also possible that the increase in Symbiodinium phylotype B and C detected in juvenile P. astreoides compared to the planulae may reflect the increase in the amount tissue used for molecular analyses; the increase in biomass may have allowed for the detection of previously undetectable phylotypes. Alternatively, the presence of phylotypes may change due to differential metabolic needs of juvenile P. astreoides during development [70].
Effects of environmental condition on symbiotic associations and juvenile growth
Although frequencies of Symbiodinium phylotype combinations in juvenile P. astreoides varied between depths, there was no significant difference in growth rates (Fig 5). This suggests that when present, phylotypes B and C behaved as background symbionts having a transitory influence on coral growth [71]. Growth may have been compensated by variation in environmental factors associated with the transplant depths where juveniles transplanted to the shallow reef were exposed to higher nutrient levels, higher light, and warmer seawater temperatures than those juveniles transplanted to the upper mesophotic. Thus a tradeoff may exist for juvenile coral growth under varying environmental conditions at the different depths which may have allowed for similar growth rates.
Juveniles reared in ex situ outdoor aquaria had significantly different frequencies of Symbiodinium phylotype combinations than those reared in in situ reef environments where the majority of juveniles hosted only phylotype A (Fig 4). Additionally, there was no difference in growth of juvenile P. astreoides from both parental depths (Fig 5). In the ex situ outdoor aquaria at BIOS (<1m), it may be advantageous for juvenile P. astreoides to only host Symbiodinium type A4 as they produce UV-protecting compounds [56, 57] in addition to physiological pathways associated with increased photoprotection [41]. These physiological traits may provide the coral competitive advantage in extreme shallow water environments. Thus, the lack of Symbiodinium diversity may be due to continuously high light levels experienced in the outdoor aquaria promoting selection for photo-protective symbiont types [41]. Alternatively, the reduced frequencies of phylotypes B and C may indicate there is greater symbiont availability in the reef environment due to the presence of adult colonies and sediments exchanging Symbiodinium into the water column. Nitschke et al. [65] found that juvenile Acropora spp. had higher Symbiodinium densities when in the presence of adult colonies and sediments as a result of coral expulsion of Symbiodinium into the water column. The higher frequencies of Symbiodinium phylotypes B and C in the in situ reared juveniles compared with those reared ex situ in aquaria may be due to increased symbiont availability on the reef in comparison to the aquaria. In addition to having significantly different frequencies of Symbiodinium phylotype combinations, the juveniles reared in outdoor aquaria grew significantly slower than those reared on the reef (Fig 4, Fig 5). This is likely due to the differences in the environments as juveniles reared in the outdoor aquaria were exposed to ambient light and temperature, making it a potentially a stressful environment in comparison to the reef.
To our knowledge, this study is among the first to examine Symbiodinium consortia through vertical transmission and early growth of a brooding coral. We document greater diversity of Symbiodinium phylotypes associated with P. astreoides throughout its depth distribution in Bermuda than previously observed [9]. However, only the dominant symbiont, Symbiodinium phylotype A4 was vertically transmitted to the majority of planulae. When juveniles were reared at different depths on the reef, the Symbiodinium consortia appeared to transition and mirror Symbiodinium phylotype combinations associated with adult P. astreoides. Juveniles transplanted to different depths had significantly different frequencies of Symbiodinium phylotype combinations but no difference in growth, suggesting potential for selection during juvenile development post transplantation. In contrast, P. astreoides juveniles reared in outdoor aquaria did not transition to mirror Symbiodinium phylotype combinations associated with adult reef P. astreoides and had slower growth rates than their reef-reared counterparts. By comparing growth and symbiotic associations of ex situ and in situ reared juveniles, this study highlights the impact of habitat type on growth and Symbiodinium consortia of juvenile corals. This is crucial for past and future interpretation of research involving juvenile corals reared in various environments. Moreover, these results document patterns of horizontal transmission of symbionts from parental P. astreoides colonies to their planulae, and variability in Symbiodinium consortia across developmental stages of juveniles reared in different environments. Taken together, this study indicates that while the relationship between P. astreoides and Symbiodinium type is conserved across reproduction, flexibility in the associated consortia during development may enable adaptation to various environments, which may aid the long-term success of the species under changing conditions.
Supporting information
Photographs are property of Kevin H. Wong (used with permission).
(EPS)
Aquaria were used to rear shallow and upper mesophotic juvenile Porites astreoides.
(EPS)
Acknowledgments
This study was supported by PADI Foundation grant #14837, a Bermuda Institute of Ocean Sciences Grant-In-Aid, funding from the Bermuda Institute of Ocean Sciences Education Department, an Albert, Norma and Howard Geller ‘77 Endowed Research Award (Clark University), and a Phycological Society of America Grant-In-Aid of Research award (all to HGR). We thank the anonymous reviewers whose comments improved the quality of this manuscript. We also thank Kevin Wong for his assistance in monitoring planulation and settlement events and Kayley You Mak for her assistance in monitoring juvenile growth. Lastly, we thank Mandy Shaler for providing the map of Bermuda.
Data availability
Sequence data, descriptions of sequence quality, and gel electrophoresis images from this study are publicly available via Clark University’s Digital Commons (http://commons.clarku.edu/facultyworks/24/).
Funding Statement
This study was funded by PADI Foundation grant #14837 (www.padifoundation.org), a Bermuda Institute of Ocean Sciences Grant-In-Aid (www.bios.edu), funding from the Bermuda Institute of Ocean Sciences Education Department (www.bios.edu), an Albert, Norma and Howard Geller ’77 Endowed Research Award (Clark University George Perkins Marsh Institute; www2.clarku.edu/departments/marsh/index.cfm), and a Phycological Society of America Grant-In-Aid of Research award (www.psaalgae.org/grants-in-aid-of-research-program). All awards were to HGR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.De’ath G, Fabricius KE, Sweatman H, Puotinen M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. PNAS. 2012; 109(44):17995–9. 10.1073/pnas.1208909109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003; 301(5635):958–60. 10.1126/science.1086050 [DOI] [PubMed] [Google Scholar]
- 3.Jackson J, Donovan M, Cramer K, Lam V. Status and trends of Caribbean coral reefs: Global Coral Reef Monitoring Network. 2014: 1970–2012. Gland, Switzerland: Global Coral Reef Monitoring Network, IUCN; 2014.
- 4.Aronson RB, Precht WF. Landscape patterns of reef coral diversity: a test of the intermediate disturbance hypothesis. J Exp Mar Biol Ecol. 1995; 192(1):1–14. [Google Scholar]
- 5.Goodbody‐Gringley G, Woollacott RM, Giribet G. Population structure and connectivity in the Atlantic scleractinian coral Montastraea cavernosa (Linnaeus, 1767). Mar Ecol. 2012; 33(1):32–48. [Google Scholar]
- 6.Serrano X, Baums IB, O'Reilly K, Smith T, Jones R, Shearer T, et al. Geographic differences in vertical connectivity in the Caribbean coral Montastraea cavernosa despite high levels of horizontal connectivity at shallow depths. Mol Ecol. 2014; 23(17):4226–40. 10.1111/mec.12861 [DOI] [PubMed] [Google Scholar]
- 7.Nunes F, Norris RD, Knowlton N. Implications of isolation and low genetic diversity in peripheral populations of an amphi-Atlantic coral. Mol Ecol. 2009; 18(20):4283–97. 10.1111/j.1365-294X.2009.04347.x [DOI] [PubMed] [Google Scholar]
- 8.Goodbody-Gringley G, Vollmer SV, Woollacott RM, Giribet G. Limited gene flow in the brooding coral Favia fragum (Esper, 1797). Mar Biol. 2010; 157(12):2591–602. [Google Scholar]
- 9.Serrano X, Baums IB, Smith TB, Jones RJ, Shearer TL, Baker AC. Long distance dispersal and vertical gene flow in the Caribbean brooding coral Porites astreoides. Sci Rep. 2016; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DH, Edmunds PJ, Carpenter RC. Increasing relative abundance of Porites astreoides on Caribbean reefs mediated by an overall decline in coral cover. Mar Ecol Prog Ser. 2008; 359:1–10. [Google Scholar]
- 11.Vermeij MJ, Bakker J, Hal Nvd, Bak RP. Juvenile coral abundance has decreased by more than 50% in only three decades on a small Caribbean island. Diversity. 2011; 3(3):296–307. [Google Scholar]
- 12.Kenkel CD, Goodbody-Gringley G, Caillaud D, Davies SW, Bartels E, Matz MV. Evidence for a host role in thermotolerance divergence between populations of the mustard hill coral (Porites astreoides) from different reef environments. Mol Ecol. 2013; 22(16):4335–48. 10.1111/mec.12391 [DOI] [PubMed] [Google Scholar]
- 13.Kenkel CD, Meyer E, Matz MV. Gene expression under chronic heat stress in populations of the mustard hill coral (Porites astreoides) from different thermal environments. Mol Ecol. 2013; 22(16):4322–34. 10.1111/mec.12390 [DOI] [PubMed] [Google Scholar]
- 14.Bak R, Engel M. Distribution, abundance and survival of juvenile hermatypic corals (Scleractinia) and the importance of life history strategies in the parent coral community. Mar Biol. 1979; 54(4):341–52. [Google Scholar]
- 15.Chornesky EA, Peters EC. Sexual reproduction and colony growth in the scleractinian coral Porites astreoides. Bio Bull. 1987; 172(2):161–77. [Google Scholar]
- 16.Byler KA, Carmi-Veal M, Fine M, Goulet TL. Multiple symbiont acquisition strategies as an adaptive mechanism in the coral Stylophora pistillata. PloS One. 2013; 8(3):e59596 10.1371/journal.pone.0059596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edmunds PJ. The effects of temperature on the growth of juvenile scleractinian corals. Mar Biol. 2008; 154(1):153–62. [Google Scholar]
- 18.Edmunds PJ, Bruno JF, Carlon DB. Effects of depth and microhabitat on growth and survivorship of juvenile corals in the Florida Keys. Mar Ecol Prog Ser. 2004; 278:115–24. [Google Scholar]
- 19.Edmunds PJ, Gates RD. Size-dependent differences in the photophysiology of the reef coral Porites astreoides. Bio Bull. 2004; 206(2):61–4. [DOI] [PubMed] [Google Scholar]
- 20.Kenkel CD, Setta SP, Matz MV. Heritable differences in fitness-related traits among populations of the mustard hill coral, Porites astreoides. Heredity. 2015; 115(6):509–16. 10.1038/hdy.2015.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantin NE, van Oppen MJ, Willis BL, Mieog JC, Negri AP. Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs. 2009; 28:405–14. [Google Scholar]
- 22.Little AF, Oppen MJ, Willis BL. Flexibility in algal symbioses shapes growth in reef corals. Science. 2004; 304(5676):1492–4. 10.1126/science.1095733 [DOI] [PubMed] [Google Scholar]
- 23.Mieog JC, Olsen JL, Berkelmans R, Bleuler-Martinez SA, Willis BL, van Oppen MJH. The roles and interactions of symbiont, host and environment in defining coral fitness. PloS One. 2009; 4(7):e6364 10.1371/journal.pone.0006364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita H, Suzuki G, Kai S, Hayashibara T, Koike K. Establishment of Coral–Algal Symbiosis Requires Attraction and Selection. PLoS One. 2014; 9(5):e97003 10.1371/journal.pone.0097003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrego D, Van Oppen M, Willis BL. Onset of algal endosymbiont specificity varies among closely related species of Acropora corals during early ontogeny. Mol Ecol. 2009; 18(16):3532–43. 10.1111/j.1365-294X.2009.04276.x [DOI] [PubMed] [Google Scholar]
- 26.Abrego D, Ulstrup KE, Willis BL, van Oppen MJ. Species-specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc R Soc Lond [Biol]. 2008; 275(1648):2273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stambler N. Zooxanthellae: the yellow symbionts inside animals Coral Reefs: an ecosystem in transition. Dordrecht: Springer Netherlands; 2011. p. 87–106. [Google Scholar]
- 28.Pochon X, Gates RD. A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai'i. Mol Phylogenet Evol. 2010; 56(1):492–7. 10.1016/j.ympev.2010.03.040 [DOI] [PubMed] [Google Scholar]
- 29.Abrego D, Willis BL, van Oppen MJ. Impact of light and temperature on the uptake of algal symbionts by coral juveniles. PloS One. 2012; 7(11):e50311 10.1371/journal.pone.0050311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker AC. Reef corals bleach to survive change. Nature. 2001; 411(6839):765–6. 10.1038/35081151 [DOI] [PubMed] [Google Scholar]
- 31.Berkelmans R, van Oppen MJH. The role of zooxanthellae in the thermal tolerance of corals: a 'nugget of hope' for coral reefs in an era of climate change. Proc R Soc Lond [Biol]. 2006; 273(1599):2305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunning R, Baker AC. Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nat Clim Chang. 2013; 3:259–62. [Google Scholar]
- 33.Silverstein RN, Cunning R, Baker AC. Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob Chang Biol. 2015; 21(1):236–49. 10.1111/gcb.12706 [DOI] [PubMed] [Google Scholar]
- 34.Stat M, Gates RD. Clade D Symbiodinium in scleractinian corals: a “nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? J Mar Biol. 2010; 2011:1–9. [Google Scholar]
- 35.Boulotte NM, Dalton SJ, Carroll AG, Harrison PL, Putnam HM, Peplow LM, et al. Exploring the Symbiodinium rare biosphere provides evidence for symbiont switching in reef-building corals. ISME J. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamashita H, Suzuki G, Hayashibara T, Koike K. Do corals select zooxanthellae by alternative discharge? Mar Biol. 2011; 158(1):87–100. [Google Scholar]
- 37.Jones A, Berkelmans R. Potential costs of acclimatization to a warmer climate: growth of a reef coral with heat tolerant vs. sensitive symbiont types. PLoS One. 2010; 5(5):e10437 10.1371/journal.pone.0010437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stat M, Morris E, Gates RD. Functional diversity in coral–dinoflagellate symbiosis. PNAS. 2008; 105(27):9256–61. 10.1073/pnas.0801328105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunning R, Gillette P, Capo T, Galvez K, Baker A. Growth tradeoffs associated with thermotolerant symbionts in the coral Pocillopora damicornis are lost in warmer oceans. Coral Reefs. 2015; 34(1):155–60. [Google Scholar]
- 40.Venn AA, Wilson MA, Rosenthal HG, Keely BJ, Douglas AE. The impact of coral bleaching on the pigment profile of the symbiotic alga, Symbiodinium. Plant Cell Environ. 2006; 29(12):2133–42. 10.1111/j.1365-3040.2006.001587.x [DOI] [PubMed] [Google Scholar]
- 41.Reynolds JM, Bruns BU, Fitt WK, Schmidt GW. Enhanced photoprotection pathways in symbiotic dinoflagellates of shallow-water corals and other cnidarians. PNAS. 2008; 105(36):13674–8. 10.1073/pnas.0805187105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thornhill DJ, LaJeunesse TC, Kemp DW, Fitt WK, Schmidt GW. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar Biol. 2006; 148(4):711–22. [Google Scholar]
- 43.Coffroth MA, Santos SR, Goulet TL. Early ontogenetic expression of specificity in a cnidarian-algal symbiosis. Mar Ecol Prog Ser. 2001; 222:85–96. [Google Scholar]
- 44.Marlow HQ, Martindale MQ. Embryonic development in two species of scleractinian coral embryos: Symbiodinium localization and mode of gastrulation. Evol Dev. 2007; 9(4):355–67. 10.1111/j.1525-142X.2007.00173.x [DOI] [PubMed] [Google Scholar]
- 45.Goodbody-Gringley G, Wetzel DL, Gillon D, Pulster E, Miller A, Ritchie KB. Toxicity of Deepwater Horizon source oil and the chemical dispersant, Corexit® 9500, to coral larvae. PloS One. 2013; 8(1):e45574 10.1371/journal.pone.0045574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johannes RE, Wiebe WJ. Method for determination of coral tissue biomass and composition. Limnol Oceanogr. 1970; 15(5):822–4. [Google Scholar]
- 47.Goodbody-Gringley G. Diel planulation by the brooding coral Favia fragum (Esper, 1797). J Exp Mar Biol Ecol. 2010; 389(1):70–4. [Google Scholar]
- 48.Pochon X, Pawlowski J, Zaninetti L, Rowan R. High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar Biol. 2001; 139(6):1069–78. [Google Scholar]
- 49.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3rd ed. Cold Spring Harbor (NY):Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 50.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997; 25(17):3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012; 28(12):1647–9. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arif C, Daniels C, Bayer T, Banguera-Hinestroza E, Barbrook A, Howe CJ, et al. Assessing Symbiodinium diversity in scleractinian corals via next‐generation sequencing‐based genotyping of the ITS2 rDNA region. Mol Ecol. 2014; 23(17):4418–33. 10.1111/mec.12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zar JH. Biostatistical analysis 4th ed. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- 54.Mitchell A, Bergmann PJ. Thermal and moisture habitat preferences do not maximize jumping performance in frogs. Funct Ecol. 2016; 30(5):733–42. [Google Scholar]
- 55.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2014. [Google Scholar]
- 56.Banaszak AT, LaJeunesse TC, Trench RK. The synthesis of mycosporine-like amino acids (MAAs) by cultured, symbiotic dinoflagellates. J Exp Mar Biol Ecol. 2000; 249(2):219–33. [DOI] [PubMed] [Google Scholar]
- 57.Banaszak AT, Santos MGB, LaJeunesse TC, Lesser MP. The distribution of mycosporine-like amino acids (MAAs) and the phylogenetic identity of symbiotic dinoflagellates in cnidarian hosts from the Mexican Caribbean. J Exp Mar Biol Ecol. 2006; 337(2):131–46. [Google Scholar]
- 58.Bongaerts P, Frade PR, Hay KB, Englebert N, Latijnhouwers KR, Bak RP, et al. Deep down on a Caribbean reef: lower mesophotic depths harbor a specialized coral-endosymbiont community. Sci Rep. 2015; 5:7652 10.1038/srep07652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodbody-Gringley G, Marchini C, Chequer AD, Goffredo S. Population structure of Montastraea cavernosa on shallow versus mesophotic reefs in Bermuda. PLoS One. 2015; 10(11):e0142427 10.1371/journal.pone.0142427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegel DA, Michaels AF, Sorensen JC, O'Brien MC, Hammer MA. Seasonal variability of light availability and utilization in the Sargasso Sea. J Geophys Res. 1995; 100(C5):8695–713. [Google Scholar]
- 61.Bongaerts P, Carmichael M, Hay KB, Tonk L, Frade PR, Hoegh-Guldberg O. Prevalent endosymbiont zonation shapes the depth distributions of scleractinian coral species. R Soc.Open Sci. 2015; 2(2):140297 10.1098/rsos.140297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warner ME, LaJeunesse TC, Robison JD, Thur RM. The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol Oceanogr. 2006; 51(4):1887–97. [Google Scholar]
- 63.Savage AM, Goodson MS, Visram S, Trapido-Rosenthal H, Wiedenmann J, Douglas AE. Molecular diversity of symbiotic algae at the latitudinal margins of their distribution: dinoflagellates of the genus Symbiodinium in corals and sea anemones. Mar Ecol Prog Ser. 2002; 244:17–26. [Google Scholar]
- 64.Dodge RE, Logan A, Antonius A. Quantitative reef assessment studies in Bermuda: a comparison of methods and preliminary results. Bull Mar Sci. 1982; 32(3):745–60. [Google Scholar]
- 65.Nitschke MR, Davy SK, Ward S. Horizontal transmission of Symbiodinium cells between adult and juvenile corals is aided by benthic sediment. Coral Reefs. 2016; 35(1)1–10. [Google Scholar]
- 66.Baker AC. Flexibility and specificity in coral-algal symbiosis: Diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst. 2003; 34:661–89. [Google Scholar]
- 67.Mieog JC, van Oppen MJH, Cantin NE, Stam WT, Olsen JL. Real-time PCR reveals a high incidence of Symbiodinium clade D at low levels in four scleractinian corals across the Great Barrier Reef: implications for symbiont shuffling. Coral Reefs. 2007; 26(3):449–57. [Google Scholar]
- 68.Holstein DM, Smith TB, Paris CB. Depth-independent reproduction in the reef coral Porites astreoides from shallow to mesophotic zones. PloS One. 2016; 11(1):e0146068 10.1371/journal.pone.0146068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Padilla-Gamiño JL, Pochon X, Bird C, Concepcion GT, Gates RD. From parent to gamete: vertical transmission of Symbiodinium (Dinophyceae) ITS2 sequence assemblages in the reef building coral Montipora capitata. PloS One. 2012; 7(6):e38440 10.1371/journal.pone.0038440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kopp C, Domart-Coulon I, Barthelemy D, Meibom A. Nutritional input from dinoflagellate symbionts in reef-building corals is minimal during planula larval life stage. Sci Adv. 2016; 29(2):255–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee MJ, Jeong HJ, Jang SH, Lee SY, Kang NS, Lee KH, et al. Most low-abundance “background” Symbiodinium spp. are transitory and have minimal functional significance for symbiotic corals. Microb Ecol. 2016; 71(3):771–83. 10.1007/s00248-015-0724-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Photographs are property of Kevin H. Wong (used with permission).
(EPS)
Aquaria were used to rear shallow and upper mesophotic juvenile Porites astreoides.
(EPS)
Data Availability Statement
Sequence data, descriptions of sequence quality, and gel electrophoresis images from this study are publicly available via Clark University’s Digital Commons (http://commons.clarku.edu/facultyworks/24/).