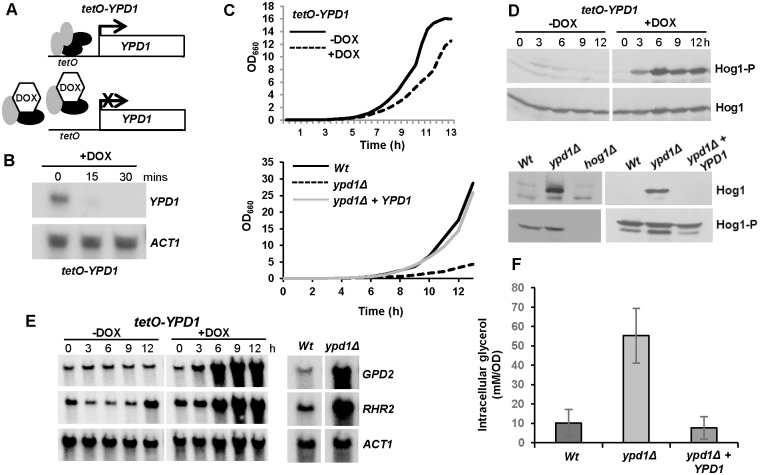
Fig 2. C. albicans cells lacking YPD1 exhibit hyperactivation of Hog1 but are viable.
(A) Strategy to control YPD1 expression. One YPD1 allele was deleted, and the remaining allele placed under the control of the E. coli tet operator (tetO) in strain THE1 to generate strain tetO-YPD1 (JC1586). THE1 cells express an E. coli tet repressor–S. cerevisiae Hap4 activation domain fusion protein. In the absence of doxycycline (DOX), this fusion protein binds as a dimer to the tet operator resulting in transcriptional activation. However, doxycycline prevents dimerisation of the fusion protein and blocks transcription. (B) Doxycycline treatment inhibits YPD1 expression. Northern analysis of YPD1 and ACT1 (control) transcript levels in tetO-YPD1 cells treated with doxycycline for the indicated times. (C) Repression or deletion of YPD1 results in a slow growth phenotype. Growth analysis of tetO-YPD1 cells, untreated or treated with doxycycline (top panel), and wild-type (Wt, JC21), ypd1Δ (JC2001) and ypd1Δ+YPD1 (JC2002) cells (bottom panel). (D) Repression or deletion of YPD1 results in constitutive phosphorylation of Hog1. Western blot analysis of whole cell extracts isolated from tetO-YPD1 cells following treatment with doxycycline for the indicated times, or from exponentially growing wild-type (Wt), ypd1Δ and ypd1Δ+YPD1 cells. Blots were probed for phosphorylated Hog1 (Hog1-P), stripped and reprobed for total Hog1 (Hog1). (E) Repression or deletion of YPD1 results in high levels of GPD2 and RHR2 expression. RNA was isolated from tetO-YPD1 cells, treated with or without doxycycline for the indicated times, or wild-type and ypd1Δ cells, and analyzed using gene-specific probes with ACT1 as a loading control. (F) Deletion of YPD1 results in increased intracellular glycerol levels. The mean ± SD is shown for 3 biological replicates.