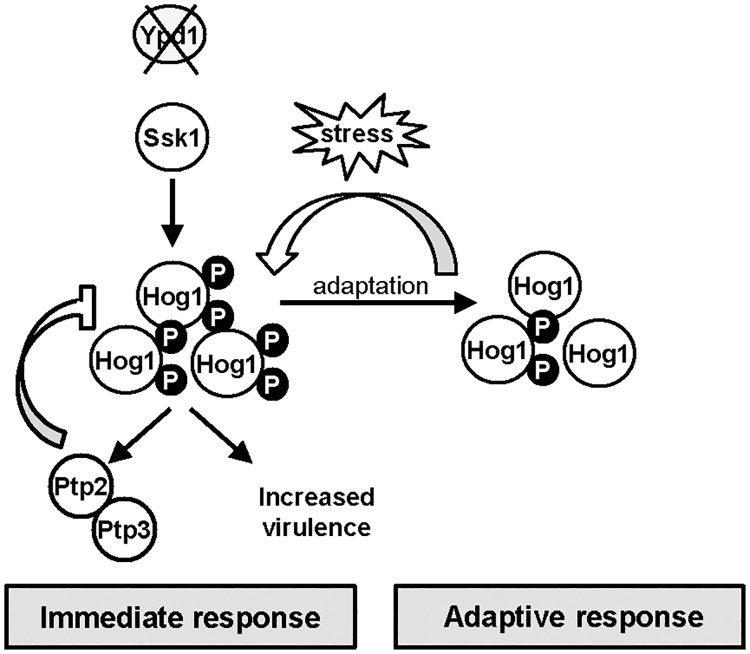
Fig 8. Ypd1 inactivation in C. albicans triggers Hog1 hyperactivation, increased virulence, and in the long term a reduction in Hog1 activity.
Model depicting outcomes following YPD1 loss in C. albicans. Loss of Ypd1 results in the accumulation of the unphosphorylated Ssk1 response regulator, which drives the activation of Hog1 under non-stressed conditions. The levels of Hog1 phosphorylation are modulated by the induction of the negative regulators Ptp2 and Ptp3 which allows cells to adapt and survive Ypd1 loss. Loss of Ypd1 function during infection increases the virulence of C. albicans, by possibly enhancing Hog1 activity promoting stress resistance and/or filamentation. Furthermore, C. albicans adapts to long-term activation of Hog1 by reducing the levels of the phosphorylated Hog1 kinase. This adaptation process prevents phenotypes associated with sustained SAPK activation and ypd1Δ cells now phenotypically resemble wild-type cells. Notably, however, this adaptation mechanism to circumvent Hog1 phosphorylation can be over-ridden following transient stress exposure and thus sustained Hog1 activation is restored.