Abstract
BACKGROUND: MUC5B is glycoprotein secreted by bronchial glands. A promoter variant in MUC5B, rs35705950, was previously found to be strongly associated with the incidence of idiopathic pulmonary fibrosis (IPF) and also the overall survival (OS) of such patients. Patients with IPF and patients with radiation pneumonitis (RP) have the similar pathologic process and clinical symptoms. However, the role of rs35705950 in patients receiving thoracic radiotherapy remains unclear. PATIENTS AND METHODS: In total, 664 patients with NSCLC receiving definitive radiotherapy (total dose ≥60 Gy) were included in our study. RP was scored via the Common Terminology Criteria for Adverse Events v3.0. OS was the second end point. MUC5B rs35705950 was genotyped, and Kaplan-Meier and Cox regression analyses were used to evaluate associations between MUC5B rs35705950 and the risk of RP or OS. RESULTS: The median patient age was 66 years (range 35-88); most (488 [73.2%]) had stage III of the disease. Until the last follow-up, 250 patients developed grade ≥ 2 RP, 82 patients developed grade ≥ 3 RP, and 440 patients died. The median mean lung dose was 17.9 Gy (range 0.15-32.74). No statistically significant associations were observed between genotypes of MUC5B rs35705950 and the incidence of RP ≥ grade 2 either in univariate analysis (hazard ratio [HR] 1.009, 95% confidence interval [CI] 0.728-1.399, P = .958) or in multivariate analysis (HR 0.921, 95% CI 0.645-1.315, P = .65). Similar results were also observed for RP ≥ grade 3, while TT/GT genotypes in MUC5B were significantly associated with poor OS in both univariate analysis (HR 1.287, 95% CI 1.009-1.640, P = .042) and multivariate analysis (HR 1.561, 95% CI 1.193-2.042, P = .001). CONCLUSION: MUC5B promoter polymorphism could be prognostic of the OS among NSCLC patients receiving definitive radiotherapy, although no significant associations were found with the risk of RP.
Introduction
Lung cancer is the leading cause of cancer-related deaths. About 85% of lung cancer is non–small cell lung cancer (NSCLC) [1]. About 80% of patients with NSCLC in the United States present with disease that is inoperable owing to local advancement or distant metastase [2]. The use of radiotherapy improves the outcome of locally advanced NSCLC, but the prognosis is still poor due to high rates of recurrence and distant metastasis. Another reason is that treatment-induced toxicities limit the dose escalation. For patients with locally advanced NSCLC, radiation-induced lung toxicity (radiation pneumonitis, RP) defines the therapeutic ratio and can also complicate the quality of life for survivors. Therefore, potentially predictive markers are needed to tailor the personalized treatment and lower the risk of treatment-induced toxicities.
MUC5B, a high-molecular glycoprotein secreted by the bronchial glands, is required for mucociliary clearance for controlling infection and maintaining the immune homeostasis in the lungs [3]. Genetic variants of MUC5B are related to various lung diseases [4], [5]. Especially a promoter variant in MUC5B, rs35705950, showed strong associations with the incidence of idiopathic pulmonary fibrosis (IPF), increased MUC5B expression, and the survival of patients with IPF [4], [6]. Association between genotypes of MUC5B rs35705950 and the risk of IPF has been widely validated in the Chinese population [7], Japanese population, German population [8], and Mexican population [9].
IPF and RP have the similar pathologic process, including persistent injuries to type II alveolar epithelial, infiltration of inflammation cells, chronic inflammation, deposition of collagen, and formation of lung fibrosis [10], [11]. Furthermore, IPF and RP also possess similar clinical symptoms, including a dry cough, shortness of breath, and chest tightness. Genotypes of MUC5B rs35705950 were significantly associated with increased risk of IPF and improved survival among patients with IPF [6]. However, the roles of MUC5B rs35705950 in NSCLC remain unclear. As far as we know, no studies have investigated the potential associations between the promoter variant of MUC5B and the incidence of RP or the survival rate in patients with NSCLC receiving definitive radiotherapy.
To address these gaps, MUC5B rs35705950 was genotyped in patients with NSCLC receiving definitive radiotherapy (total dose ≥ 60Gy). Our hypothesis was that genotypes of MUC5B rs35705950 were associated with the incidence of RP and overall survival (OS) in the studied population.
Patients and Methods
Study Population
This retrospective analysis was approved by the institutional review board of The University of Texas MD Anderson Cancer Center, and we complied with all applicable Health Insurance Portability and Accountability Act regulations. We searched an institutional database to identify patients who had received definitive radiotherapy for NSCLC at MD Anderson from 1999 through 2014 who had 1) histologically confirmed NSCLC; 2) received a total radiation dose of ≥60 Gy [or ≥60 Gy (RBE) for proton therapy]; 3) available computed tomography or positron emission tomography scans obtained within 1 year of completing radiotherapy, to be used for detecting and scoring RP; and 4) available archived blood samples for genotyping. Patients who had received stereotactic ablative radiotherapy were excluded. A total of 664 patients met these criteria and were the subjects of this analysis. The concurrent chemotherapy regimen was carboplatin and paclitaxel weekly during the radiotherapy.
RP was assessed at each follow-up visit after the completion of radiotherapy and graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events version 3.0. Follow-up visits took place within the first 1 to 3 months after radiotherapy and then every 3 months after that for 2 years; those visits included interval history and physical examinations and imaging studies.
Genotyping Methods
Genomic DNA was extracted from the buffy coat fraction of blood samples using a blood DNA mini kit (Qiagen, Inc.) according to the manufacturer's instructions. Spectrophotometric absorbance determined the purity and concentration of the DNA at 260 and 280 nm. The original extracted genomic DNA was diluted into 5-ng/μl aliquots for genotyping with Taqman real-time polymerase chain reaction. Primers and probes were from Applied Biosystems. Amplification was done under the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. For all genotypes, the assay success rate was >95%, and concordance of repeated sample testing was 100%.
Statistical Analyses
Potential associations between RP risk and genotypes were assessed with a Cox proportional hazard model, with consideration of time to the event. Propensity score was estimated via SPSS 22 and used for the adjustment of the confounders in the multivariate cox regression. Kaplan-Meier analysis was used to assess the effect of different genotypes on the cumulative probability of RP. All of these analyses were done with SPSS 22. All tests were two-sided, and differences were considered significant at P < .05.
Results
Patient Characteristics
Characteristics of the study population are shown in Table 1. The median age of the patients was 66 years (range 35-88 years), and most (488 [73.2%]) had stage III NSCLC. The median gross tumor volume (GTV) was 94.8 cm3 (range 1.5-1271.5 cm3), the median radiation dose was 69 Gy (range 60-87.5 Gy), and the median mean lung dose (MLD) was 17.9 Gy (range 0.15-32.741 Gy). Radiation was delivered as proton beam therapy to 139 patients (20.8%), as intensity-modulated (photon) radiotherapy to 331 (49.6%), and as three-dimensional conformal radiotherapy to 174 (26.1%). Besides, 242 patients (36.3%) received induction chemotherapy, and 560 patients (84.0%) received concurrent chemotherapy. In our study, the frequency of the rare allele TT genotypes was only 2%.
Table 1.
Patient Characteristics
| Characteristic | All | Percentage |
|---|---|---|
| Age, years | ||
| <66 (median) | 332 | 50 |
| ≥66 | 332 | 50 |
| Sex | ||
| Male | 363 | 55 |
| Female | 301 | 45 |
| Race | ||
| White | 569 | 86 |
| Other | 95 | 14 |
| Disease stage | ||
| I-IIIA | 296 | 41 |
| IIIB, IV, recurrence | 333 | 59 |
| Tumor histology | ||
| Adenocarcinoma | 293 | 44 |
| SCC and other | 371 | 56 |
| Karnofsky Performance Status score | ||
| <80 | 101 | 15 |
| ≥80 | 563 | 85 |
| Induction chemotherapy | ||
| No | 417 | 63 |
| Yes | 247 | 37 |
| Smoking status | ||
| Never | 55 | 8 |
| Former/current | 597 | 92 |
| Total radiation dose, Gy | ||
| <69.03 (median) | 328 | 50 |
| ≥69.03 | 330 | 50 |
| GTV, cm3 | ||
| <95.2 (median) | 306 | 50 |
| ≥95.2 | 305 | 50 |
| MLD, Gy | ||
| <17.9 (median) | 320 | 50 |
| ≥17.9 | 319 | 50 |
| Radiation modality | ||
| Photon (X-ray) | 511 | 77 |
| Proton | 139 | 23 |
| MUCB rs35705950 | ||
| GG | 517 | 81 |
| GT | 103 | 16 |
| TT | 15 | 2 |
Association between MUC5B rs35705950 and the Incidence of RP
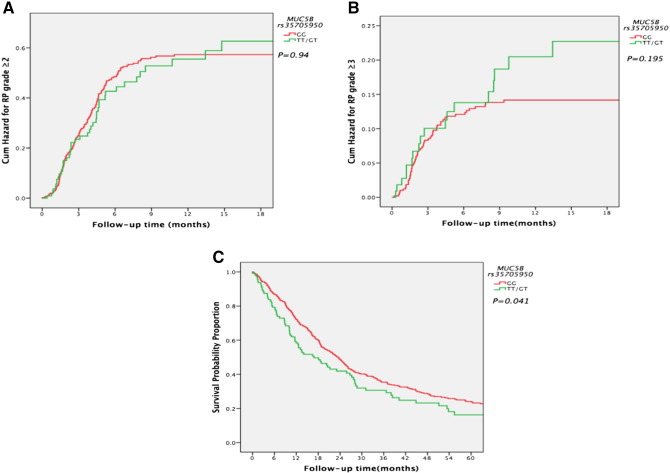
Propensity score analysis was used for the adjustment of confounders in the multivariate analysis. Similar to other studies, MLD was strongly associated with the incidence of RP ≥ grade 2 in the patients receiving definitive radiotherapy in both univariate (hazard ratio [HR] 2.08, 95% confidence interval [CI] 1.601-2.703, P = .001) and multivariate analyses (HR 1.883, 95% CI 1.402-2.528, P < .001). However, the analysis presented in Table 2 did not show statistically significant associations between genotypes of MUC5B rs35705950 and incidence of RP ≥ grade 2 either in univariate analysis (HR 1.009, 95% CI 0.728-1.399, P = .958) or in multivariate analysis (HR 0.921, 95% CI 0.645-1.315, P = .65), nor was a significant association shown in the K-M analysis (Figure 1A, P = .94). Potential associations between the incidence of severe RP (≥grade 3) and MUC5B rs35705950 were analyzed. Similar to the results for RP ≥ grade 2, the results of analysis presented in Table 3 and Figure 1B did not show statistically significant associations between genotypes of MUC5B rs35705950 and incidence of RP ≥ grade 3 either in univariate analysis (HR 1.009, 95% CI 0.728-1.399, P = .958) or in multivariate analysis (HR 1.614, 95% CI 0.92-2.831, P = .095). No statistically significant difference was shown in the K-M analysis (Figure 1B, P = .195).
Table 2.
Stepwise Univariate and Multivariate Cox Regression Analyses to Identify Predictors of Grade ≥ 2 RP
| Characteristics | Univariate Analysis (RP ≥ grade 2) |
Multivariate Analysis (RP ≥ grade 2)⁎ |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (≥66 vs <66) | 1.236 | 0.964-1.585 | .095 | 1.361 | 1.038-1.784 | .026 |
| Gender (male vs female) | 1.005 | 0.784-1.290 | .966 | 0.882 | 0.673-1.155 | .361 |
| Race (black and other vs white) | 1.053 | 0.742-1.493 | .773 | 0.876 | 0.605-1.267 | .481 |
| Disease stage (IIIB, IV, recurrence vs I-IIIA) | 1.010 | 0.782-1.303 | .941 | 0.857 | 0.653-1.126 | .269 |
| Histology (SCC & other vs Adn) | 0.954 | 0.743-1.224 | .711 | 0.982 | 0.749-1.287 | .894 |
| KPS (≥80 vs <80) | 0.771 | 0.554-1.075 | .125 | 0.687 | 0.481-0.98 | .038 |
| Concurrent chemotherapy (yes vs no) | 1.102 | 0.739-1.644 | .633 | 0.966 | 0.609-1.532 | .966 |
| Smoking status (current/former vs never) | 0.825 | 0.532-1.279 | .39 | 0.769 | 0.474-1.248 | .288 |
| Total dose (≥69.03 vs <69.03 Gy) | 0.799 | 0.622-1.026 | .079 | 0.83 | 0.63-1.093 | .184 |
| GTV (≥95.19 vs <95.19 cm3) | 1.539 | 1.186-1.999 | .001 | 1.299 | 0.977-1.726 | .072 |
| MLD (≥17.93 vs <17.93 Gy) | 2.08 | 1.601-2.703 | .001 | 1.883 | 1.402-2.528 | <.001 |
| Technique (proton vs 3D-CRT + IMRT) | 0.932 | 0.688-1.263 | .65 | 1.105 | 0.794-1.537 | .555 |
| Muc5b rs35705950 (TT/GT vs GG) | 1.009 | 0.728-1.399 | .958 | 0.921 | 0.645-1.315 | .65 |
Abbreviations: NI, not included; KPS, Karnofsky Performance Status score; SCC, squamous cell carcinoma; 3D-CRT, 3-dimensional conformal (photon) radiation therapy; IMRT, intensity-modulated (photon) radiation therapy.
The propensity score was used for adjustments of confounders in the multivariate analysis.
Figure 1.
(A) Cumulative probability of grade ≥ 2 RP in 664 patients with NSCLC according to MUC5B genotypes: GG/GT versus TT. (B) Cumulative probability of grade ≥ 3 RP in 664 patients with NSCLC according to MUC5B genotypes: GG/GT versus TT. (C) Cumulative probability of OS in 664 patients with NSCLC according to MUC5B genotypes: GG/GT versus TT.
Table 3.
Stepwise Univariate and Multivariate Cox Regression Analyses to Identify Predictors of Grade ≥ 3 RP
| Characteristics | Univariate Analysis (RP ≥ grade 3) |
Multivariate Analysis (RP ≥ grade 3)⁎ |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (≥66 vs <66) | 1.545 | 0.993-2.401 | .054 | 1.502 | 0.944-2.39 | .086 |
| Gender (male vs female) | 1.188 | 0.764-1.848 | .443 | 0.919 | 0.574-1.472 | .726 |
| Race (black and other vs white) | 1.048 | 0.568-1.934 | .881 | 0.74 | 0.388-1.411 | .36 |
| Disease stage (IIIB, IV, recurrence vs I-IIIA) | 0.779 | 0.499-1.217 | .273 | 0.696 | 0.437-1.107 | .126 |
| Histology (SCC & other vs Adn) | 0.968 | 0.625-1.498 | .883 | 0.969 | 0.606-1.549 | .894 |
| KPS (≥80 vs <80) | 0.51 | 0.305-0.852 | .01 | 0.416 | 0.24-0.722 | .002 |
| Concurrent chemotherapy (yes vs no) | 1.032 | 0.516-2.064 | .928 | 1.24 | 0.537-2.866 | .614 |
| Smoking status (current/former vs never) | 1.123 | 0.489-2.580 | .784 | 0.834 | 0.333-2.088 | .698 |
| Total dose (≥69.03 vs <69.03 Gy) | 0.657 | 0.421-1.026 | .065 | 0.758 | 0.468-1.227 | .26 |
| GTV (≥95.19 vs <95.19 cm3) | 2.76 | 1.678-4.539 | <.001 | 2.124 | 1.262-3.577 | .005 |
| MLD (≥17.93vs <17.93 Gy) | 3.922 | 2.318-6.639 | <.001 | 3.844 | 2.1-7.038 | <.001 |
| Technique (proton vs 3D-CRT + IMRT) | 0.65 | 0.359-1.176 | .154 | 1.395 | 0.823-2.363 | .216 |
| Muc5b rs35705950 (TT/GT vs GG) | 1.009 | 0.728-1.399 | .958 | 1.614 | 0.92-2.831 | .095 |
The propensity score was used for adjustments of confounders in the multivariate analysis.
Association between MUC5B rs35705950 and OS in the Studied Population
As shown in Table 4, concurrent chemotherapy was obviously associated with improved OS in the studied population in univariate analysis (HR 0.702, 95% CI 0.541-0.911, P = .008), whereas GTV was significantly associated with poor OS in both univariate analysis (HR 1.776, 95% CI 1.474-2.169, P < .001) and multivariate analysis (HR 1.633, 95% CI 1.323-2.016, P < .001). Analysis in Table 4 suggests that compared to genotypes GG, TT/GT genotypes in MUC5B were associated with poor OS in both univariate analysis (HR 1.287, 95% CI 1.009-1.640, P = .042) and multivariate analysis (HR 1.561, 95% CI 1.193-2.042, P = .001). The K-M analysis also showed similar results (Figure 1C, P = .041).
Table 4.
Stepwise Univariate and Multivariate Cox Regression Analyses to Identify Predictors of OS in the Studied Population
| Characteristics | Univariate Analysis (OS) |
Multivariate Analysis (OS)⁎ |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age (≥66 vs <66) | 1.289 | 1.068-1.555 | .008 | 1.219 | 0.993-1.497 | .059 |
| Gender (male vs female) | 1.279 | 1.058-1.547 | .011 | 1.13 | 0.916-1.394 | .252 |
| Race (black and other vs white) | 1.127 | 0.892-1.424 | .315 | 0.998 | 0.762-1.306 | .986 |
| Disease stage (IIIB, IV, recurrence vs I-IIIA) | 1.081 | 0.894-1.308 | .422 | 1.1 | 0.897-1.35 | .361 |
| Histology (SCC & other vs Adn) | 1.268 | 1.047-1.535 | .015 | 1.175 | 0.953-1.449 | .132 |
| KPS (≥80 vs <80) | 0.72 | 0.565-0.918 | .008 | 0.854 | 0.655-1.114 | .245 |
| Concurrent chemotherapy (yes vs no) | 0.702 | 0.541-0.911 | .008 | 0.782 | 0.577-1.059 | .112 |
| Smoking status (current/former vs never) | 1.146 | 0.807-1.627 | .447 | 1.011 | 0.691-1.479 | .955 |
| Total dose (≥69.03vs <69.03 Gy) | 0.864 | 0.715-1.043 | .864 | 1.036 | 0.838-1.281 | .744 |
| GTV (≥95.19 vs <95.19 cm3) | 1.776 | 1.454-2.169 | <.001 | 1.633 | 1.323-2.016 | <.001 |
| MLD (≥17.93 vs <17.93 Gy) | 1.541 | 1.27-1.871 | <.001 | 1.459 | 1.183-1.798 | <.001 |
| Technique (proton vs 3D-CRT + IMRT) | 0.788 | 0.611-1.016 | .066 | 0.983 | 0.740-1.306 | .907 |
| Muc5b rs35705950 (TT/GT vs GG) | 1.287 | 1.009-1.640 | .042 | 1.561 | 1.193-2.042 | .001 |
The propensity score was used for adjustments of confounders in the multivariate analysis.
Discussion
Our study suggests that the pulmonary fibrosis–associated MUC5B promoter polymorphism did not influence the incidence of RP but was statistically significantly associated with OS among patients receiving definitive radiotherapy for NSCLC.
RP and IPF have similar pathogenic changes and are both with final formation of pulmonary fibrosis. Common mechanisms underlying pulmonary fibrosis are the wound-healing responses, including injury, inflammation, and repair. Chronic inflammation could lead to an imbalance in the production of chemokines, cytokines, and growth factors, and disrupt cellular recruitment. These changes, coupled with excessive profibrotic factors, could turn a well-controlled healing response into a pathogenic fibrotic response [12]. Different from the previous findings in patients with IPF [4], [13], our study did not show significant associations between the MUC5B promoter polymorphism and the incidence of RP in patients with NSCLC. Similar to our results, the MUC5B promoter polymorphism was not associated with the incidence of interstitial pneumonia among patients with systemic sclerosis [14]. RP mainly develops in patients with thoracic cancer receiving high-dose thoracic radiotherapy, whereas the causes of IPF are unknown. Therefore, the differences may be due to the genetic heterogeneity of different diseases.
There are only a few studies about the roles of MUC5B in lung cancer. Our study found that the MUC5B promoter polymorphism rs35705950 was significantly associated with the prognosis of patients receiving definitive radiotherapy for NSCLC. This result is biologically reasonable. Early in the year of 1996, expression of MUC5B was found to be significantly associated with a high risk of distant metastasis in patients with NSCLC [15]. The previous study also found that expression of MUC5B was apparently associated with poor OS in patients with NSCLC [16], although the expression of MUC5B was thought to be a favorable marker for the OS in EGFR mutant NSCLC [17]. The variant allele of MUC5B was related to increased expression of MUC5B [4], [18]. Therefore, the effect of the MUC5B promoter polymorphism on OS in patients with NSCLC may be through the expression of MUC5B. The MUC5B promoter polymorphism is prognostic of the OS in patients receiving radiotherapy for NSCLC and will be helpful to personalize the treatment for patients with NSCLC in the future.
The strengths of our study included relatively large sample size and comprehensive follow-up information. As far as we know, this is the first study investigating the roles of MUC5B promoter polymorphism rs35705950 on radiation-induced lung injury and OS in patients with NSCLC. Our study also had some limitations. It was retrospective, and we did not test the expression of MUC5B. Expression of MUC5B and its role in NSCLC will be analyzed in the future.
In summary, we found no significant associations between the MUC5B promoter polymorphism rs35705950 and incidence of RP. The MUC5B promoter polymorphism rs35705950 was apparently associated with OS in our studied group. We are currently validating these results with samples from patients enrolled in an ongoing prospective trial of definitive radiotherapy for NSCLC.
Ethical Approval and Consent to Participate
This retrospective analysis was approved by the institutional review board of The University of Texas MD Anderson Cancer Center, and we complied with all applicable Health Insurance Portability and Accountability Act regulations. Written consent form was obtained from each patient.
Consent for Publication
No individual data were included in our study.
Availability of Data and Supporting Materials Section
Please contact author for data requests.
Funding
Not applicable.
Authors' Contribution
X. L. Y. and Z. X. L.: contributed to the study conception and design, and the drafting, review, and final approval of the manuscript.
J. Y. and T. X.: contributed to the acquisition and analysis of data and the review and final approval of the manuscript.
J. Y. and Y. P. S.: contributed to the experimental performance.
J. Y., D. G., M. J., and L. L.: contributed to the acquisition and analysis of data and the review and final approval of the manuscript.
S. H.: contributed to the acquisition of data, and in reviewing and final approval of the manuscript.
Acknowledgements
Ju Yang was supported by the China Scholarship Council to complete the project in the University of Texas MD Anderson Cancer Center.
Footnotes
Ju Yang was supported by the China Scholarship Council to complete the project in the University of Texas MD Anderson Cancer Center.
Translational Relevance: The etiology of pulmonary fibrotic diseases is various, including exposure to allergens, chemicals, radiation, and environmental particles. The cause of idiopathic pulmonary fibrosis (IPF) is still unclear, whereas radiation pneumonitis (RP) is caused by radiation-induced lung injury. Association between genotypes of MUC5B rs35705950 and the risk of IPF has been widely validated. In this study, we tried to figure out roles of MUC5B rs35705950 in the risk of RP or the prognosis of patients with non–small cell lung cancer (NSCLC) receiving definitive radiotherapy. Although our study suggested that there were no significant associations between MUC5B rs35705950 and the incidence of RP in our studied group, MUC5B rs35705950 was strongly related with overall survival. Therefore, MUC5B rs35705950 may be prognostic of the outcome of patients with NSCLC. Our results will be helpful to identify patients with poor outcome and to individualize the treatments of patients with NSCLC.
Conflict of interest: The authors do not see any conflicts of interest.
Please contact author for data requests.
Contributor Information
Ju Yang, Email: yangjutjh@163.com.
Ting Xu, Email: TXu@mdanderson.org.
Daniel R. Gomez, Email: DGomez@mdanderson.org.
Melenda Jeter, Email: mdjeter@mdanderson.org.
Lawrence B Levy, Email: lblevy@mdanderson.org.
Yipeng Song, Email: syp1972@sina.com.
Stephen Hahn, Email: SHahn@mdanderson.org.
Zhongxing Liao, Email: zliao@mdanderson.org, zliao@mdanderon.org.
Xianglin Yuan, Email: yxl@medmail.com.cn.
References
- 1.Bender E. Epidemiology: the dominant malignancy. Nature. 2014;513:S2–S3. doi: 10.1038/513S2a. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamio K, Matsushita I, Hijikata M, Kobashi Y, Tanaka G, Nakata K, Taguchi Y, Homma S, Nakata K, Azuma A. Promoter analysis and aberrant expression of the MUC5B gene in diffuse panbronchiolitis. Am J Respir Crit Care Med. 2005;171:949–957. doi: 10.1164/rccm.200409-1168OC. [DOI] [PubMed] [Google Scholar]
- 6.Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, Silveira LJ, Lindell KO, Steele MP, Loyd JE. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309:2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang H, Hu Y, Shang L, Li Y, Yang L, Chen Y. Association between MUC5B polymorphism and susceptibility and severity of idiopathic pulmonary fibrosis. Int J Clin Exp Pathol. 2015;8:14953–14958. [PMC free article] [PubMed] [Google Scholar]
- 8.Horimasu Y, Ohshimo S, Bonella F, Tanaka S, Ishikawa N, Hattori N, Kohno N, Guzman J, Costabel U. MUC5B promoter polymorphism in Japanese patients with idiopathic pulmonary fibrosis. Respirology. 2015;20:439–444. doi: 10.1111/resp.12466. [DOI] [PubMed] [Google Scholar]
- 9.Peljto AL, Selman M, Kim DS, Murphy E, Tucker L, Pardo A, Lee JS, Ji W, Schwarz MI, Yang IV. The MUC5B promoter polymorphism is associated with idiopathic pulmonary fibrosis in a Mexican cohort but is rare among Asian ancestries. Chest. 2015;147:460–464. doi: 10.1378/chest.14-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trott KR, Herrmann T, Kasper M. Target cells in radiation pneumopathy. Int J Radiat Oncol Biol Phys. 2004;58:463–469. doi: 10.1016/j.ijrobp.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 11.Gunther A, Korfei M, Mahavadi P, von der Beck D, Ruppert C, Markart P. Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis. Eur Respir Rev. 2012;21:152–160. doi: 10.1183/09059180.00001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu QQ, Zhang XL, Zhang SM, Zhang SW, Min HY, Yi L, Xu B, Song Y. Association between the MUC5B Promoter polymorphism rs35705950 and idiopathic pulmonary fibrosis: a meta-analysis and trial sequential analysis in Caucasian and Asian populations. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peljto AL, Steele MP, Fingerlin TE, Hinchcliff ME, Murphy E, Podlusky S, Carns M, Schwarz M, Varga J, Schwarz DA. The pulmonary fibrosis-associated MUC5B promoter polymorphism does not influence the development of interstitial pneumonia in systemic sclerosis. Chest. 2012;142:1584–1588. doi: 10.1378/chest.12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu CJ, Yang PC, Shun CT, Lee YC, Kuo SH, Luh KT. Overexpression of MUC5 genes is associated with early post-operative metastasis in non–small-cell lung cancer. Int J Cancer. 1996;69:457–465. doi: 10.1002/(SICI)1097-0215(19961220)69:6<457::AID-IJC7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Nagashio R, Ueda J, Ryuge S, Nakashima H, Jiang SX, Kobayashi M, Yanagita K, Katono K, Satoh Y, Masuda N. Diagnostic and prognostic significances of MUC5B and TTF-1 expressions in resected non–small cell lung cancer. Sci Rep. 2015;5:8649. doi: 10.1038/srep08649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakata K, Tsuchiya T, Tomoshige K, Takagi K, Yamasaki N, Matsumoto K, Miyazaki T, Nanashima A, Whitsett JA, Maeda Y. A favourable prognostic marker for EGFR mutant non–small cell lung cancer: immunohistochemical analysis of MUC5B. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loh AX, Johnson L, Ng W, Swallow DM. Cis-acting allelic variation in MUC5B mRNA expression is associated with different promoter haplotypes. Ann Hum Genet. 2010;74:498–505. doi: 10.1111/j.1469-1809.2010.00613.x. [DOI] [PubMed] [Google Scholar]