Abstract
Understanding multilevel predictors of colorectal cancer (CRC) screening test modality can help inform screening program design and implementation. We used North Carolina Medicare, Medicaid, and private, commercially available, health plan insurance claims data from 2003 to 2008 to ascertain CRC test modality among people who received CRC screening around their 50th birthday, when guidelines recommend that screening should commence for normal risk individuals. We ascertained receipt of colonoscopy, fecal occult blood test (FOBT) and fecal immunochemical test (FIT) from billing codes. Person-level and county-level contextual variables were included in multilevel random intercepts models to understand predictors of CRC test modality, stratified by insurance type.
Of 12,570 publicly-insured persons turning 50 during the study period who received CRC testing, 57% received colonoscopy, whereas 43% received FOBT/FIT, with significant regional variation. In multivariable models, females with public insurance had lower odds of colonoscopy than males (odds ratio [OR] = 0.68; p < 0.05). Of 56,151 privately-insured persons turning 50 years old who received CRC testing, 42% received colonoscopy, whereas 58% received FOBT/FIT, with significant regional variation. In multivariable models, females with private insurance had lower odds of colonoscopy than males (OR = 0.43; p < 0.05). People living 10–15 miles away from endoscopy facilities also had lower odds of colonoscopy than those living within 5 miles (OR = 0.91; p < 0.05).
Both colonoscopy and FOBT/FIT are widely used in North Carolina among insured persons newly age-eligible for screening. The high level of FOBT/FIT use among privately insured persons and women suggests that renewed emphasis on FOBT/FIT as a viable screening alternative to colonoscopy may be important.
Keywords: Colorectal cancer, Colonoscopy, Fecal occult blood test, Cancer screening tests, Medicaid, Medicare, Multilevel analysis
Highlights
-
•
Claims data were used to assess predictors of colorectal cancer screening modality.
-
•
Both patient and regional characteristics were predictive of modality chosen.
-
•
Fecal testing was associated with private insurance, being female, and longer distance to endoscopy center.
-
•
Fecal testing represents an important, less invasive, alternative to colonoscopy.
1. Introduction
Colorectal cancer (CRC) screening is a proven strategy to reduce colon cancer morbidity and mortality when used according to guidelines by average-risk people ages 50–75 years old. When used as recommended by guidelines, CRC screening reduces the chances of developing and dying from CRC (US Preventive Services Task Force, 2008). Screening for clinically undetectable pre-cancers and cancers among average-risk men and women can be performed by several modalities, including: colonoscopy once every 10 years; fecal occult blood test (FOBT) or fecal immunochemical test (FIT) every year; or flexible sigmoidoscopy once every 5 years (Levin et al., 2008). In practice, colonoscopy and FOBT/FIT are the most widely used screening tests, with differing sensitivity and specificity, preparation procedures, invasiveness, recovery time, and follow-up procedures recommended (US Preventive Services Task Force, 2008, Centers for Disease Control and Prevention, 2013).
Since 2000, the US has seen an increase in CRC screening (Klabunde et al., 2011, Meissner et al., 2006, Kim et al., 2005, Centers for Disease Control and Prevention, 2011) with variation in choice of test modality over time. Specifically, there has been a notable increase in colonoscopy uptake (Meissner et al., 2006, Kim et al., 2005) and a slight decrease in fecal testing uptake (Klabunde et al., 2011, Centers for Disease Control and Prevention, 2011, Steele et al., 2013, Bandi et al., 2012). While no single factor caused this shift in screening modality, between 1998 and 2001, Medicare and state General Assemblies implemented policy changes that required insurers to reimburse providers at least in part for the cost of colonoscopy screening, along with other types of CRC screening, for age-eligible or high-risk persons (Kim et al., 2005, Centers for Medicare and Medicaid Services, CMS, 2009). These policy changes may have incentivized providers to recommend screening with colonoscopy more often due to higher reimbursement and may have motivated patients to be screened via colonoscopy due to reduced out-of-pocket cost and longer duration of coverage (Klabunde et al., 2011).
Physician recommendation and patient preferences factor into choice of CRC screening modality (Hawley et al., 2014, Inadomi et al., 2012). Some physicians have been noted to prefer and recommend colonoscopies over FOBT/FIT for screening (Bandi et al., 2012; Hawley et al., 2014, Inadomi et al., 2012, Schwartz, 2004, Reed et al., 2008, Pruitt et al., 2014, Shariff-Marco et al., 2013). Patient preferences differ widely across populations, with some evidence suggesting that preference for colonoscopy is associated with family history of CRC and desire for accuracy, whereas preference for fecal testing may be associated with desire for ease, lower cost, and convenience (Meissner et al., 2006, Steele et al., 2013, Towne et al., 2014). Racial variation in screening modality indicates that non-Hispanic Whites receive colonoscopies more often (Steele et al., 2013, Inadomi et al., 2012). Latinos and non-Hispanic Blacks prefer FOBT over colonoscopy (Klabunde et al., 2011, Steele et al., 2013, Bandi et al., 2012, Inadomi et al., 2012, Towne et al., 2014), despite low levels of overall CRC screening among both of these groups (Klabunde et al., 2011). Although individual-level factors explain much of the variation in screening modality (Pruitt et al., 2014, Shariff-Marco et al., 2013), area-level resources have been associated with screening modality in some studies (Shariff-Marco et al., 2013), and geographic location and access to screening are significantly associated with screening choices among some racial/ethnic minority populations (Towne et al., 2014). These observations prompt a need to further evaluate geographic and area-level factors in order to more clearly understand how individual and geographic factors simultaneously influence CRC screening modality received by certain populations.
Given continued variation in patient preferences and physician recommendation, as well as the need for a more in-depth examination of area-level factors, we sought to gain a better understanding of geographic variation in, and the specific predictors of, CRC screening modality (colonoscopy versus FOBT/FIT) among average-risk people who were incident screeners—that is, newly age-eligible for CRC screening. We focused on incident screeners because these individuals would not be expected to have a prior history of CRC testing that may affect choice of modality. We were interested in the first CRC test modality received. We therefore investigated these questions among publicly and privately insured people in North Carolina (NC) who received CRC testing around their 50th birthday, the age screening is recommended to commence by the United States Preventive Services Task Force (USPSTF) for normal risk individuals. We focused on NC because it is a large, racially, socioeconomically, and geographically heterogeneous state with high CRC mortality and is an ideal setting in which to compare CRC testing from linked claims data to national self-reported data. Self-report accuracy is higher among individuals receiving colonoscopies than for FOBT/FIT (Dodou & de Winter, 2014), perhaps due to the substantial preparation and recovery time required for colonoscopy compared to FOBT/FIT. Such recall bias may potentially result in misleading assessments of the balance among modalities. Therefore, assessing CRC modality using data other than self-reported information is essential.
2. Methods
2.1. Data
We used NC Medicare and Medicaid fee-for-service insurance claims data from people insured by either or both of these public insurance providers in 2003–2008, inclusive. We also used fee-for-service claims data from private, commercially available health plans in NC in 2003–2008, inclusive. We required continuous enrollment to ensure that we were able to fully capture receipt of CRC testing from health insurance claims.
2.2. Study population
In accordance with the USPSTF guidelines for initiation of CRC screening at 50 years old, we included people who turned 50 at any time during the study period and received at least one CRC screening test. Among Medicaid and Medicare enrollees, due to eligibility criteria, this primarily represents a disabled population, whereas this age group in the privately insured population primarily represents working adults receiving employer-sponsored insurance. Although Medicare covers screening colonoscopy or FOBT/FIT without coinsurance or copay according to recommended testing schedules (Centers for Medicare and Medicaid Services, CMS, 2015), state Medicaid programs and private insurance plans vary in their CRC screening coverage policies. We included people who had received either colonoscopy, FOBT or FIT during the study period (14,787 publicly, 59,875 privately insured), as this represents approximately 96% of CRC screening tests performed in this population (Wheeler et al., 2014). We also limited our study population to those individuals who remained alive and did not move to a different county during the study period so that we could understand regional variation in modality and examine the effect of area-level factors, such as distance from patient residence to an endoscopy provider, on CRC testing modality (2183 publicly, 2813 privately insured were excluded). Because we were interested in distance to endoscopy centers as a measure of geographic access, we further excluded a small number of people without valid ZIP code data (3 publicly, 769 privately insured). Finally, we also excluded people with prior history of CRC or colectomy (31 publicly, 142 privately insured), as defined in the available claims data, to ensure that our measures reflected screening rather than surveillance procedures.
2.3. Dependent variable
CRC testing was defined as beneficiary receipt of colonoscopy or FOBT/FIT in the claims during the study period. Receipt of colonoscopy was defined as our binary, dependent variable (receipt of FOBT/FIT was the reference category). Billing codes used are listed in Supplemental Table 1 (Online). Codes included procedures performed for screening and diagnostic intent, since these cannot be reliably distinguished in claims data because billing practices vary across institutions (Schenck et al., 2008, Schenck et al., 2007).
For people who received both procedures, the first procedure received was designated as the primary outcome for analysis because it more likely aligns with initial choice of modality. For example, an initial abnormal FOBT/FIT would require follow-up colonoscopy, but this second procedure was not included in our analysis.
2.4. Independent variables
Both person-level characteristics and county-level contextual variables were included in the analysis. Due to differences in data sources and variable availability, we were not able to include the same person-level variables in both the publicly and privately insured analyses. For example, marital status is only available in the privately insured sample, whereas race is only available in the publicly insured sample. Variables such as gender, year patient turned age 50, and approximate distance between patients' residence and the nearest endoscopy facility, were included in both the publicly and privately insured analyses.
Distance to nearest endoscopy facility was measured based on beneficiaries' residential ZIP code and endoscopy facilities' ZIP codes. We identified all endoscopy facilities in NC using the 2007 State Medical Facilities Plan (SMFP) Inventory of Endoscopy Rooms in Licensed Facilities, in which we identified 178 facilities across NC's 100 counties. We measured the straight line distance between all beneficiaries' residential ZIP code centroids and all the ZIP code centroids of facilities' locations using ArcGIS 10.1.(ESRI) We used straight-line distance as a proxy for geographic access to endoscopy in the absence of more granular data on public transit and other transportation resources; prior studies have suggested that straight-line distance is > 90% correlated with driving distance and time (Boscoe et al., 2012). We ranked these distances and then identified the nearest endoscopy facility to each beneficiary, employing a previously published approach (Wheeler et al., 2012, Wheeler et al., 2014). Distances to nearest endoscopy facility were then categorized into 5 groups: < 5 miles, 5 to < 10 miles, 10 to < 15 miles, 15 to < 20 miles, 20 + miles. In addition, we also included the total endoscopy procedures performed in each of the endoscopy facilities to create a county-level measure of total endoscopy procedural volume per 10,000 population. Counties without any endoscopy center as reported by the SMFP (N = 28) were assigned a value of 0 for endoscopy procedural volume.
County-level variables were obtained from the 2005 Area Resource File (ARF). These included total count of all general practitioners, percent living under the federal poverty line, percent with less than a high school education, percent unemployed, percent uninsured (age 40–64), and percent non-white in the county, as defined by ARF. Most of these variables were categorized into quartiles excepting count of medical generalists, which was dichotomized at the median.
2.5. Statistical analyses
Our models were stratified by public and private insurance because we anticipated that the magnitude and significance of predictors would vary by payer and because some control variables were not available across all payers. Among the publicly insured, we also examined predictors of modality by Medicare only, Medicaid only, and dually enrolled (Medicare and Medicaid) in a secondary analysis. We first conducted bivariate analyses using Chi-squared tests to examine unadjusted relationships between CRC testing modality and independent variables. For multivariate analysis, we used multilevel logistic models to examine probability of receiving colonoscopy versus FOBT/FIT, adjusting for individual-level and county-level characteristics. In these models, we allowed the county intercept to be random in order to account for correlated data among the individuals who lived in the same county.
We obtained average predicted probabilities of colonoscopy for each county in NC from the final multilevel models. We present the geographic variation using choropleth maps in which the predicted probability of colonoscopy was grouped across 100 counties and used cut-off points comparable for both public and private insurance groups. We also included the location of endoscopy facilities in the map. All analyses were conducted in 2015, using SAS software (version 9.3; SAS Institute, Inc., Cary, NC). Maps were created using ArcMap of the ArcGIS Desktop Release 10 (ESRI). This study was approved by the UNC Institutional Review Board.
3. Results
3.1. Publicly insured sample
We identified 12,570 publicly insured persons turning 50 years old who received colorectal cancer testing via colonoscopy or FOBT/FIT; 57% of these had a colonoscopy procedure whereas 43% received FOBT/FIT. In bivariable analyses (Table 1), males were more likely to receive colonoscopy than females (63% versus 54%). People with higher likelihood of receiving colonoscopy included those turning 50 in an earlier year (60% in 2003 versus 52% in 2008), and living in areas with low unemployment rate (60% in low versus 55% in high quartile).
Table 1.
Sample Characteristics for Publicly and Privately Insured Beneficiaries in North Carolina (2003–2008).
| Publicly insured N = 12,570 |
Privately insured N = 56,151 |
|||
|---|---|---|---|---|
| Colo. N = 7196 (57%) |
FOBT/FIT N = 5374 (43%) |
Colo. N = 23,321 (42%) |
FOBT/FIT N = 32,830 (58%) |
|
| N(%) | N(%) | N(%) | N(%) | |
| Gender | ||||
| Male | 2886 (63) |
1668 (37) |
10,643 (54) |
8889 (46) |
| Female | 4310 (54) |
3706 (46) |
12,678 (35) |
23,941 (65) |
| Race | N/A | N/A | ||
| White | 4081 (58) |
2983 (42) |
||
| Black | 2757 (57) |
2065 (43) |
||
| Other | 358 (52) |
326 (48) |
||
| Marital status | N/A | N/A | ||
| Married | 16,264 (41) |
23,338 (59) |
||
| Not married | 5136 (42) |
7028 (58) |
||
| Unknown | 1921 (44) |
2464 (56) |
||
| Ever in SEHP | N/A | N/A | ||
| Yes | 13,066 (41) |
19,044 (59) |
||
| No | 10,255 (43) |
13,786 (57) |
||
| Public insurance type | N/A | N/A | ||
| Medicare | 1982 (61) |
1289 (39) |
||
| Medicaid | 1559 (54) |
1341 (46) |
||
| Dual | 3655 (57) |
2744 (43) |
||
| Year turned 50 | ||||
| 2003 | 1506 (60) |
1007 (40) |
4896 (44) |
6249 (56) |
| 2004 | 1385 (58) |
1007 (42) |
4703 (44) |
5978 (56) |
| 2005 | 1232 (57) |
913 (43) |
4315 (43) |
5681 (57) |
| 2006 | 1250 (59) |
867 (41) |
3927 (42) |
5336 (58) |
| 2007 | 1018 (55) |
823 (45) |
3298 (40) |
5010 (60) |
| 2008 | 805 (52) |
757 (48) |
2182 (32) |
4576 (68) |
| Distance to the nearest endoscopy facility | ||||
| < 5 miles | 3291 (57) |
2488 (43) |
11,426 (42) |
15,479 (58) |
| 5–10 miles | 2050 (58) |
1485 (42) |
6934 (41) |
9813 (59) |
| 10–15 miles | 1106 (57) |
839 (43) |
3286 (39) |
5115 (61) |
| 15–20 miles | 480 (55) |
393 (45) |
1061 (41) |
1528 (59) |
| 20 + miles | 269 (61) |
169 (39) |
614 (41) |
895 (59) |
| Number of generalists (per 10,000) | ||||
| Below median | 2841 (56) |
2220 (44) |
6851 (38) |
11,001 (62) |
| Above median | 4355 (58) |
3154 (42) |
16,470 (43) |
21,829 (57) |
| Endoscopy facility test volume (per 10,000) | ||||
| 0 | 722 (57) |
549 (43) |
1610 (40) |
2407 (60) |
| 1–199 | 504 (58) |
366 (42) |
1672 (41) |
2417 (59) |
| 200–399 | 2031 (56) |
1606 (44) |
7674 (40) |
11,338 (60) |
| 400–599 | 1544 (61) |
1001 (39) |
4393 (42) |
6171 (58) |
| 600–799 | 1022 (56) |
801 (44) |
3341 (42) |
4526 (58) |
| 800 + | 1373 (57) |
1051 (43) |
4631 (44) |
5971 (56) |
| Quartiles of regional uninsurance (40–64 years old) | ||||
| Low | 3146 (59) |
2224 (41) |
9661 (42) |
13,088 (58) |
| Low-medium | 2316 (56) |
1855 (44) |
8299 (41) |
11,781 (59) |
| Medium-high | 1117 (57) |
847 (43) |
3439 (40) |
5249 (60) |
| High | 617 (58) |
448 (42) |
1922 (41) |
2712 (59) |
| Quartiles of regional % non-white | ||||
| Low | 906 (56) |
725 (44) |
2832 (41) |
4011 (59) |
| Low-medium | 1712 (56) |
1359 (44) |
5629 (38) |
9040 (62) |
| Medium-high | 2175 (59) |
1539 (41) |
9106 (41) |
13,029 (59) |
| High | 2403 (58) |
1751 (42) |
5754 (46) |
6750 (54) |
| Quartiles of regional unemployment rate | ||||
| Low | 1634 (60) |
1090 (40) |
8654 (43) |
11,286 (57) |
| Low-medium | 2012 (59) |
1422 (41) |
6908 (42) |
9665 (58) |
| Medium-high | 2007 (56) |
1585 (44) |
5328 (40) |
7931 (60) |
| High | 1543 (55) |
1277 (45) |
2431 (38) |
3948 (62) |
Notes: Colo: colonoscopy; FOBT: fecal occult blood test; FIT: fecal immunohistochemical test; SEHP: State Employees Health Plan.
In multivariable models (Table 2), after adjusting for person-level and county-level contextual factors, females with public insurance had lower odds of receiving colonoscopy versus FOBT/FIT (odds ratio [OR] = 0.68) than males. Those with Medicaid or dual insurance had lower odds of using colonoscopy (OR = 0.84 and 0.90, respectively) compared to Medicare beneficiaries. Across types of public insurance (Table 3), statistically significant effects of county-level unemployment were observed for both Medicare-only and Medicaid-only beneficiaries, with people living in areas with higher unemployment associated with lower odds of receiving colonoscopy (versus FOBT/FIT) as compared to people in areas with lower unemployment. Statistically significant effects of distance to endoscopy and county-level non-white population proportion also were observed in the Medicare-only population. Beneficiaries living 10 – 15 miles or more than 20 miles away from endoscopy facilities were more likely to receive colonoscopy (versus FOBT/FIT) as compared to those who lived within 5 miles of an endoscopy facility. Medicare-only beneficiaries living in areas with more non-white population had a higher likelihood of receiving colonoscopy (versus FOBT/FIT), compared to those living in areas with less non-white population.
Table 2.
Multilevel logit model results: odds ratios for colonoscopy (versus FOBT/FIT) among people turning 50 years old who were tested in North Carolina (2003–2008), by insurance type.
| Effect | Publicly insured |
Privately insured |
||||
|---|---|---|---|---|---|---|
| (N = 12,570) |
(N = 56,151) |
|||||
| OR | LB | UB | OR | LB | UB | |
| Gender (female vs male, ref = male) | 0.68 | 0.63 | 0.74 | 0.43 | 0.39 | 0.49 |
| Race | N/A | |||||
| Black vs white | 0.97 | 0.89 | 1.05 | |||
| Other vs white | 0.93 | 0.78 | 1.11 | |||
| Insurance type | N/A | |||||
| Dual vs Medicare | 0.90 | 0.83 | 0.99 | |||
| Medicaid vs Medicare | 0.84 | 0.75 | 0.94 | |||
| Year turned 50 | ||||||
| 2003 vs 2008 | 1.37 | 1.21 | 1.57 | 1.61 | 1.49 | 1.74 |
| 2004 vs 2008 | 1.27 | 1.11 | 1.45 | 1.62 | 1.51 | 1.75 |
| 2005 vs 2008 | 1.26 | 1.11 | 1.45 | 1.57 | 1.47 | 1.68 |
| 2006 vs 2008 | 1.34 | 1.17 | 1.53 | 1.53 | 1.43 | 1.63 |
| 2007 vs 2008 | 1.17 | 1.02 | 1.35 | 1.38 | 1.30 | 1.48 |
| Distance to the nearest endoscopy facility | ||||||
| 5–10 vs < 5 miles | 1.10 | 1.00 | 1.21 | 1.00 | 0.94 | 1.06 |
| 10–15 vs < 5 miles | 1.06 | 0.94 | 1.19 | 0.91 | 0.85 | 0.98 |
| 15–20 vs < 5 miles | 1.05 | 0.88 | 1.25 | 0.96 | 0.82 | 1.14 |
| 20 + vs < 5 miles | 1.30 | 0.99 | 1.72 | 0.85 | 0.62 | 1.15 |
| Marital status | N/A | |||||
| Married vs not married | 0.98 | 0.94 | 1.01 | |||
| Unknown vs not married | 1.06 | 0.95 | 1.18 | |||
| Ever in SEHP (yes vs no; ref = no) | N/A | 1.10 | 1.05 | 1.15 | ||
| Endoscopy facility test volume (per 10,000) | ||||||
| 1–199 vs 0 | 1.08 | 0.78 | 1.49 | 1.06 | 0.80 | 1.42 |
| 200–399 vs 0 | 1.03 | 0.79 | 1.36 | 1.00 | 0.78 | 1.28 |
| 400–599 vs 0 | 1.29 | 0.96 | 1.75 | 0.90 | 0.64 | 1.26 |
| 600–799 vs 0 | 1.16 | 0.83 | 1.61 | 1.05 | 0.73 | 1.49 |
| 800 + vs 0 | 1.12 | 0.79 | 1.58 | 0.97 | 0.68 | 1.40 |
| Number of generalists (above median vs below median) | 0.96 | 0.80 | 1.15 | 1.12 | 0.94 | 1.35 |
| Regional uninsurance (40–64) | ||||||
| Low-medium vs low | 0.89 | 0.70 | 1.13 | 1.12 | 0.84 | 1.49 |
| Medium-high vs low | 0.93 | 0.71 | 1.23 | 1.13 | 0.82 | 1.56 |
| High vs low | 0.99 | 0.72 | 1.34 | 1.15 | 0.82 | 1.61 |
| Regional % non-white | ||||||
| Low-medium vs low | 1.09 | 0.84 | 1.42 | 0.99 | 0.78 | 1.26 |
| Medium-high vs low | 1.32 | 1.01 | 1.72 | 1.08 | 0.85 | 1.36 |
| High vs low | 1.31 | 0.98 | 1.76 | 1.40 | 1.04 | 1.88 |
| Regional unemployment rate | ||||||
| Low-medium vs low | 0.83 | 0.65 | 1.05 | 0.78 | 0.62 | 0.97 |
| Medium-high vs low | 0.82 | 0.65 | 1.03 | 0.88 | 0.71 | 1.10 |
| High vs low | 0.85 | 0.65 | 1.11 | 0.82 | 0.64 | 1.06 |
Notes: Statistically significant differences highlighted in bold print. FOBT: fecal occult blood test; FIT: fecal immunohistochemical test; OR: odds ratio; LB: lower bound; UB: upper bound; N/A: not applicable; SEHP: State Employees Health Plan.
Table 3.
Multilevel logit model results: Odds ratios for colonoscopy (versus FOBT/FIT) among people turning 50 years old who were tested in North Carolina (2003–2008) for all publicly insured, by insurance type.
| Effect | Medicare only |
Medicaid only |
Dual |
||||||
|---|---|---|---|---|---|---|---|---|---|
| (N = 3271) |
(N = 2900) |
(N = 6399) |
|||||||
| OR | LB | UB | OR | LB | UB | OR | LB | UB | |
| Gender (female vs male, ref = male) | 0.65 | 0.55 | 0.76 | 0.66 | 0.56 | 0.78 | 0.70 | 0.62 | 0.80 |
| Race | |||||||||
| Black vs white | 1.15 | 0.96 | 1.37 | 0.83 | 0.65 | 1.05 | 0.96 | 0.84 | 1.11 |
| Other vs white | 0.90 | 0.60 | 1.35 | 1.01 | 0.78 | 1.31 | 0.61 | 0.45 | 0.82 |
| Year turned 50 | |||||||||
| 2003 vs 2008 | 1.34 | 1.06 | 1.68 | 1.43 | 1.07 | 1.91 | 1.41 | 1.16 | 1.72 |
| 2004 vs 2008 | 1.35 | 1.06 | 1.72 | 1.65 | 1.27 | 2.13 | 1.11 | 0.92 | 1.34 |
| 2005 vs 2008 | 1.30 | 0.99 | 1.70 | 1.44 | 1.06 | 1.96 | 1.18 | 0.97 | 1.42 |
| 2006 vs 2008 | 1.51 | 1.18 | 1.93 | 1.58 | 1.20 | 2.07 | 1.19 | 0.98 | 1.45 |
| 2007 vs 2008 | 1.35 | 1.02 | 1.79 | 1.38 | 1.03 | 1.85 | 1.03 | 0.86 | 1.24 |
| Distance to the nearest endoscopy facility | |||||||||
| 5–10 vs < 5 miles | 1.12 | 0.94 | 1.33 | 1.08 | 0.89 | 1.30 | 1.12 | 0.97 | 1.30 |
| 10–15 vs < 5 miles | 1.26 | 1.01 | 1.57 | 1.15 | 0.91 | 1.47 | 0.93 | 0.77 | 1.13 |
| 15–20 vs < 5 miles | 1.11 | 0.80 | 1.52 | 0.98 | 0.64 | 1.50 | 1.05 | 0.84 | 1.30 |
| 20 + vs < 5 miles | 2.65 | 1.55 | 4.53 | 1.11 | 0.69 | 1.81 | 1.03 | 0.66 | 1.63 |
| Endoscopy facility test volume (per 10,000) | |||||||||
| 1–199 vs 0 | 1.29 | 0.86 | 1.94 | 0.76 | 0.47 | 1.23 | 1.13 | 0.74 | 1.73 |
| 200–399 vs 0 | 1.31 | 0.93 | 1.85 | 0.72 | 0.49 | 1.07 | 1.09 | 0.78 | 1.54 |
| 400–599 vs 0 | 1.58 | 1.06 | 2.37 | 0.84 | 0.54 | 1.33 | 1.42 | 0.96 | 2.12 |
| 600–799 vs 0 | 1.52 | 0.98 | 2.33 | 0.73 | 0.44 | 1.20 | 1.16 | 0.73 | 1.85 |
| 800 + vs 0 | 1.18 | 0.79 | 1.74 | 0.67 | 0.42 | 1.06 | 1.32 | 0.86 | 2.03 |
| Number of generalist (above median vs below median) | 0.88 | 0.71 | 1.08 | 1.13 | 0.87 | 1.46 | 0.92 | 0.73 | 1.15 |
| Regional uninsurance (40–64) | |||||||||
| Low-medium vs low | 0.84 | 0.62 | 1.12 | 0.93 | 0.70 | 1.24 | 0.83 | 0.63 | 1.08 |
| Medium-high vs low | 0.93 | 0.71 | 1.22 | 0.63 | 0.43 | 0.92 | 1.02 | 0.72 | 1.44 |
| High vs low | 0.83 | 0.62 | 1.13 | 0.78 | 0.54 | 1.13 | 1.12 | 0.80 | 1.58 |
| Regional % non-white | |||||||||
| Low-medium vs low | 1.25 | 0.93 | 1.69 | 1.14 | 0.84 | 1.56 | 0.95 | 0.69 | 1.32 |
| Medium-high vs low | 1.38 | 1.10 | 1.74 | 1.29 | 0.90 | 1.86 | 1.25 | 0.86 | 1.80 |
| High vs low | 1.59 | 1.18 | 2.16 | 1.10 | 0.81 | 1.50 | 1.23 | 0.87 | 1.74 |
| Regional unemployment rate | |||||||||
| Low-medium vs low | 0.80 | 0.61 | 1.04 | 1.01 | 0.74 | 1.36 | 0.86 | 0.62 | 1.17 |
| Medium-high vs low | 0.74 | 0.59 | 0.92 | 0.73 | 0.56 | 0.95 | 0.90 | 0.68 | 1.18 |
| High vs low | 0.66 | 0.49 | 0.89 | 0.78 | 0.58 | 1.06 | 0.91 | 0.69 | 1.20 |
Notes: Statistically significant differences highlighted in bold print. FOBT: fecal occult blood test; FIT: fecal immunohistochemical test; OR: odds ratio; LB: lower bound; UB: upper bound; N/A: not applicable; SEHP: State Employees Health Plan.
3.2. Privately insured sample
We identified 56,151 privately insured persons turning 50 years old in this study who received colorectal cancer testing via colonoscopy or FOBT/FIT during our study period. Overall, 42% of the privately insured had a colonoscopy procedure as their first CRC test and 58% FOBT/FIT (Table 1). In bivariable analyses, a higher proportion of males received colonoscopy as their first CRC test than females (54% versus 35%, Table 1). Additionally, people more likely to receive colonoscopy included: those living in areas with above the average numbers of generalists (43% versus 38%), high proportion of non-white residents (46% in high versus 38% for low-medium quartile), and low unemployment (43% in low versus 38% for high quartile).
In multivariable models (Table 2), after adjusting for person-level and county-level contextual factors, females with private insurance had lower odds of colonoscopy versus FOBT/FIT (odds ratio = 0.43, Table 2). People turning 50 in earlier years than 2008 were 38–62% more likely to receive colonoscopy versus FOBT/FIT. People living 10–15 miles away from endoscopy facilities had slightly lower odds of receiving colonoscopy than those living within 5 miles of an endoscopy facility (OR = 0.91). Regionally, privately insured people living in areas with the highest percentage of non-white residents had higher odds of colonoscopy, compared to the lowest quartile of non-white residents (OR = 1.4).
3.3. Geographic variation
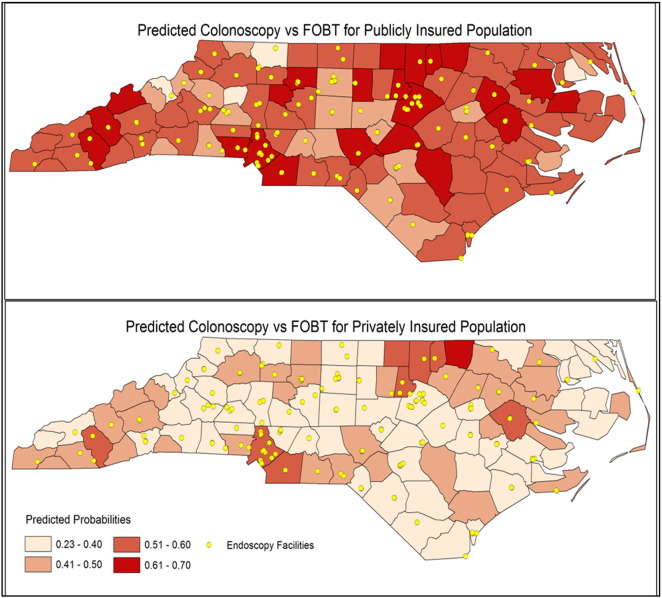
Fig. 1 shows, across NC counties, the publicly insured had a higher probability of receiving colonoscopy than the privately insured sample. Within both publicly and privately insured, significant county-level variation in CRC test modality was observed, with the highest levels of colonoscopy use observed in the North Central and South Central parts of the state.
Fig. 1.
Geographic Variation of Model Predicted Probability of Colonoscopy over FOBT/FIT across 100 Counties.
Notes: Maps stratified by insurance type (public versus private) indicate county-specific, fully adjusted predicted probabilities of colonoscopy testing use (as opposed to FOBT/FIT) among people turning 50 years old who were tested for CRC in 2003–2008. Yellow dots indicate endoscopy center locations across the state. Publicly insured persons were more likely to receive colonoscopy than FOBT/FIT, compared to privately insured persons. Across both insurance types, the highest colonoscopy use was observed in the North Central county region and in the South Central (Charlotte metropolitan) area. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We used a novel multipayer claims dataset representing approximately 70,000 unique persons to ascertain CRC testing modality (colonoscopy versus FOBT/FIT) among people turning 50 years old who were newly eligible for CRC screening and received at least one CRC test during our study period. We found that a substantial number of people (43–58%) received FOBT/FIT as their initial or incident CRC test during this period, indicating that this modality represents an important alternative to the more invasive colonoscopy for many people. In particular, our data suggest that FOBT/FIT is widely used among privately insured and women. Our overall findings are consistent with Wernli and colleagues who found 60–70% of FOBT/FIT and 20–30% of colonoscopy use in 2002–2010 among members of Group Health age 50 (Wernli et al., 2014). Thus, fecal testing represents an important alternative to the more invasive and expensive colonoscopy for many people in NC. Previous studies indicate that patients desire having CRC screening choices and appreciate their providers informing them of all relevant alternatives in such decisions (Hawley et al., 2014, Inadomi et al., 2012). Therefore, providers need to fully inform eligible patients of all relevant test alternatives and empower them to make an informed decision about CRC screening consistent with their preferences.
At first glance, it appears that our data contradict National Health Interview Survey (NHIS) (Centers for Disease Control and Prevention, 2013) and the Behavioral Risk Factor Surveillance System (BRFSS) self-reported data (which suggest that colonoscopy is the primary modality used among 50–75 year olds at 61.7% overall, compared to FOBT/FIT at 10.4% overall) (Klabunde et al., 2013). However, it is important to recognize that BRFSS data are measuring a different outcome—prevalence of CRC screening modality as it relates to the entire age-eligible population, rather than modality used in incident CRC testing around the age of 50. In addition, because FOBT/FITs confer screening coverage for only one year, whereas colonoscopies cover patients for 10 years, we expect to see a higher number of FOBT/FITs in a 6-year cross-section of CRC testing patterns. Lastly, self-reported data are known to be differentially mis-measured by CRC test modality (with FOBT/FIT more often being under-reported) (Dodou & de Winter, 2014). Because our study used insurance claims data, we have access to an arguably more objective measure of colonoscopy/FOBT/FIT balance (Bradbury et al., 2005). Nevertheless, claims data are also an imperfect resource, in that only procedures billed are measurable; therefore, it is possible that our analysis may not have captured all screening procedures performed in this sample if insurance claims were not filed (e.g., some FOBT/FITs may be missed since they are inexpensive to perform) (Schenck et al., 2008). However, because colonoscopy is an expensive procedure and FOBT/FIT is relatively inexpensive, we believe that under-ascertainment of colonoscopy would be rare (i.e., self-pay would be unlikely) and under-ascertainment of FOBT/FIT, if it occurs, would only further sway the testing balance toward greater use of FOBT/FIT.
Compared to national BRFSS data, our analysis showed greater regional variation in CRC test modality across NC than has been observed across states (Klabunde et al., 2013, Xu et al., 2013). NC is a diverse state in terms of rurality/urbanicity and access to health care resources with substantial regional variation in overall rates of CRC testing use (Wheeler et al., 2014). Observed variation in CRC test modality may be due to differential insurance generosity, regional differences in access to and use of primary care, or differential provider recommendations and referral patterns regarding FOBT/FIT and colonoscopy. In our privately insured population, FOBT/FIT was the dominant CRC test modality at 58%. This finding may reflect privately insured persons foregoing colonoscopy as a first screening test due to more significant cost-sharing burden or longer waiting times, which may drive people to use fecal tests. In contrast, the higher prevalence of colonoscopies in the publicly insured group may reflect provider preferences for colonoscopy among publicly insured persons, who may be perceived as less compliant with annual FOBT/FIT. Colonoscopy was received more often by men, which may reflect provider recommendations (in light of evidence showing that women are more adherent to annual FOBT/FIT screening than colonoscopy) and/or women's preferences for FOBT/FIT due to concerns about bowel prep and test inconvenience associated with colonoscopy (Chacko et al., 2015). Our data point to interesting and important future qualitative and quantitative research that is needed to better understand whether these differences in test modality result from provider preferences or perceptions of patients, reimbursement-driven incentives, patient preferences or personal risk perception, or some combination of these.
4.1. Limitations
Whether the CRC test received was for screening versus symptom-driven purposes is not altogether clear from claims (Schenck et al., 2008, Schenck et al., 2007). Consistent with best practices (Schenck et al., 2007, Schenck et al., 2008), we have included both screening and diagnostic procedures in our analysis. It is possible that in-office FOBTs done by providers after a rectal exam may have been included in our FOBT measure (as opposed to fecal screening done at home). Importantly, from a population health perspective, regardless of the intent or location of the test, any CRC testing helps to reduce the risk of CRC-related morbidity and mortality. In addition, our data may not be generalizable to uninsured persons, people with transient insurance, those living outside NC, or different age groups. Our results were limited to the years 2003–2008, due, in part, to delays in billing processing that slow the release of some claims data. As such, our data may not represent more contemporary trends in CRC testing, but they nevertheless provide historical trends which may be used to predict future utilization of CRC testing modalities (i.e., suggesting increasing uptake of FOBT/FIT). Finally, some variables such as race and marital status were not available across all payers in the analysis, which limited our ability to directly compare the effects of such person-specific measures on CRC screening outcomes and to fully control for the same potential confounders across payers. Although we opted to include these variables to the extent possible in our analyses because we hypothesized a priori that they may affect CRC screening modality, our multivariable findings indicated race and marital status were not statistically significant predictors of test modality in the publicly and privately insured samples, respectively. Therefore, we believe that the unavailability of these measures across all payers did not meaningfully affect our results and conclusions.
4.2. Conclusion
We present an objective portrayal of CRC testing practices from a variety of insured persons, providing a more complete understanding of CRC testing modality use than has previously been described in self-reported, single payer, and/or single institution studies. We anticipate lessons learned from our analysis regarding the continued use of FOBT/FIT may be useful to NC public health officials, as well as other states and settings, planning CRC screening programs. Although both colonoscopy and FOBT/FIT are being used in cancer prevention efforts in NC, our data and others' suggest that renewed emphasis on FOBT/FIT may have enhanced utility and could result in increased screening rates in hard-to-reach populations such as persons who experience greater system-level barriers to care (e.g., Medicaid enrollees) (Wernli et al., 2014, Pignone et al., 2014). For example, these results are currently being used in partnership with county health departments and Community Care of NC to implement a mailed reminder plus FIT kit quality improvement intervention among Medicaid enrollees living in NC counties with low CRC screening rates. Importantly, however, fecal-based testing will require annual re-screening and additional efforts to track and schedule re-screening. In NC and elsewhere, providing patients with CRC screening test options, monitoring screening activity through electronic record systems, using automated systems to issue screening reminders, fully informing patients of test characteristics and preparation procedures, and ensuring that patient preferences are respected, will likely result in advancements toward national goals of increasing CRC screening, such as the Healthy People 2020 target of having 70.5% of persons screened for CRC (Office of Disease Prevention and Health Promotion ODPHP, 2015) and the National Colorectal Cancer Roundtable goal of having 80% of people screened by 2018 (National Colorectal Cancer Roundtable, 2015).
The following are the supplementary data related to this article.
Colorectal cancer testing modality description and billing codes.
Acknowledgements
This research was funded, in part, by the Centers for Disease Control and Prevention (CDC) Special Interest Project entitled “Behavioral economics of colorectal cancer screening in underserved populations” [CDC-SIP-11-041, Co-PIs: Pignone and Wheeler] and the CDC and National Cancer Institute (NCI)-funded Cancer Prevention and Control Research Network [1-U48-DP005017-01, PI: Wheeler]. In addition: SBW was further supported by the Agency for Healthcare Research and Quality (AHRQ), 1-K-12 HS019468-01 Mentored Clinical Scientists Comparative Effectiveness Development Award (PI: Weinberger; Scholar: Wheeler). SBW was also supported as a faculty trainee through the Carolina Community Network Center to Reduce Cancer Health Disparities (CCN II), funded by theNCI Center to Reduce Cancer Health Disparities through its Community Network Program Centers [U54-CA153602]. MPP was further supported by the National Institutes of Health (NIH), K05 CA129166 Established Investigator Award in Cancer Prevention and Control: Improving Cancer-Related Patient Decision Making (PI: Pignone); MEM was supported by the National Science Foundation (NSF), CMMI-1433602 (PI: Mayorga); and, claims data and analytic support were provided by the Integrated Cancer Information and Surveillance System (ICISS), a UNC Lineberger Comprehensive Cancer Center resource funded by the state of North Carolina through the University Cancer Research Fund as well as a pilot grant from the North Carolina Translational and Clinical Sciences Institute through the National Center for Advancing Translational Sciences (NCATS), NIH [Grant Award Number 1UL1TR001111] (PI: Hassmiller Lich).
References
- Bandi P., Cokkinides V., Smith R.A., Jemal A. Trends in colorectal cancer screening with home-based fecal occult blood tests in adults ages 50 to 64 years, 2000–2008. Cancer. 2012;118(20):5092–5099. doi: 10.1002/cncr.27529. [DOI] [PubMed] [Google Scholar]
- Boscoe F.P., Henry K.A., Zdeb M.S. A nationwide comparison of driving distance versus straight-line distance to hospitals. Prof. Geogr. 2012;64(2):188–196. doi: 10.1080/00330124.2011.583586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury B.D., Brooks D.R., Brawarsky P., Mucci L.A. Test-retest reliability of colorectal testing questions on the Massachusetts Behavioral Risk Factor Surveillance System (BRFSS) Prev. Med. 2005;41(1):303–311. doi: 10.1016/j.ypmed.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Vital signs: colorectal cancer screening, incidence, and mortality—United States, 2002-2010. MMWR Morb. Mortal. Wkly Rep. 2011;60(26):884–889. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Vital signs: colorectal cancer screening test use. MMWR. 2013;62(44):883. [Google Scholar]
- Centers for Medicare and Medicaid Services, CMS National Coverage Determination (NCD) for colorectal cancer screening tests (210.3). Baltimore (MD) 2009. http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=281&ncdver=3&NCAId=212&NcaName=Screening+DNA+Stool+Test+for+Colorectal+Cancer&IsPopup=y&bc=AAAAAAAACAAAAA%3D%3D& (Publication Number 100-3. Available from:) (Accessed: Sep 29, 2014) [PubMed]
- Centers for Medicare and Medicaid Services, CMS Colorectal cancer screenings. 2015. https://www.medicare.gov/coverage/colorectal-cancer-screenings.html ([Baltimore (MD) Available From:) (Accessed: Aug 7)
- Chacko L., Macaron C., Burke C.A. Colorectal cancer screening and prevention in women. Dig. Dis. Sci. 2015;60(3):698–710. doi: 10.1007/s10620-014-3452-4. [DOI] [PubMed] [Google Scholar]
- Dodou D., de Winter J.C. Agreement between self-reported and registered colorectal cancer screening: a meta-analysis. Euro. J. Cancer Care (Engl). 2014 doi: 10.1111/ecc.12204. [DOI] [PubMed] [Google Scholar]
- ESRI ArcGIS Desktop [computer program]. Version 93 Environmental System Research Institute.
- Hawley S., Lillie S., Cooper G., Elston Lafata J. Managed care patients' preferences, physician recommendations, and colon cancer screening. Am. J. Manag. Care. 2014;20(7):555–561. [PMC free article] [PubMed] [Google Scholar]
- Inadomi J.M., Vijan S., Janz N.K. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch. Intern. Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.A., Porterfield D., Gizlice Z. Trends in up-to-date status in colorectal cancer screening, North Carolina, 1998–2002. N. C. Med. J. 2005;66(6):420–426. [PubMed] [Google Scholar]
- Klabunde C.N., Cronin K.A., Breen N. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol. Biomark. Prev. 2011;20(8):1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde C.N., Joseph D.A., King J.B., White A., Plescia M. Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb. Mortal. Wkly Rep. 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- Levin B., Lieberman D.A., McFarland B. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Meissner H.I., Breen N., Klabunde C.N., Vernon S.W. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol. Biomark. Prev. 2006;15(2):389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- National Colorectal Cancer Roundtable Tools & resources – 80% by 2018. 2015. http://nccrt.org/tools/80-percent-by-2018/ (Available from:) (Accessed: Mar, 11)
- Office of Disease Prevention and Health Promotion ODPHP . Office of Disease Prevention and Health Promotion. 2015. Healthy People 2020. (Available from: https://www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives. Accessed: Sept 30, 2014) [Google Scholar]
- Pignone M.P., Crutchfield T.M., Brown P.M. Using a discrete choice experiment to inform the design of programs to promote colon cancer screening for vulnerable populations in North Carolina. BMC Health Serv. Res. 2014;14(1):1. doi: 10.1186/s12913-014-0611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt S.L., Leonard T., Zhang S., Schootman M., Halm E.A., Gupta S. Physicians, clinics, and neighborhoods: multiple levels of influence on colorectal cancer screening. Cancer Epidemiol. Biomark. Prev. 2014;23(7):1346–1355. doi: 10.1158/1055-9965.EPI-13-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A.E., Mikels J.A., Simon K.I. Older adults prefer less choice than younger adults. Psychol. Aging. 2008;23(3):671–675. doi: 10.1037/a0012772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck A.P., Klabunde C.N., Warren J.L. Data sources for measuring colorectal endoscopy use among Medicare enrollees. Cancer Epidemiol. Biomark. Prev. 2007;16(10):2118–2127. doi: 10.1158/1055-9965.EPI-07-0123. [DOI] [PubMed] [Google Scholar]
- Schenck A.P., Klabunde C.N., Warren J.L. Evaluation of claims, medical records, and self-report for measuring fecal occult blood testing among medicare enrollees in fee for service. Cancer Epidemiol. Biomark. Prev. 2008;17(4):799–804. doi: 10.1158/1055-9965.EPI-07-2620. [DOI] [PubMed] [Google Scholar]
- Schwartz B. Ecco; 2004. The Paradox of Choice: Why More Is Less. [Google Scholar]
- Shariff-Marco S., Breen N., Stinchcomb D.G., Klabunde C.N. Multilevel predictors of colorectal cancer screening use in California. Am. J. Manag. Care. 2013;19(3):205–216. [PMC free article] [PubMed] [Google Scholar]
- Steele C.B., Rim S.H., Joseph D.A., King J.B., Seeff L.C. Colorectal cancer incidence and screening - United States, 2008 and 2010. MMWR Surveill. Summ. 2013;62(Suppl. 3):53–60. [PubMed] [Google Scholar]
- Towne S.D., Jr., Smith M.L., Ory M.G. Geographic variations in access and utilization of cancer screening services: examining disparities among American Indian and Alaska native elders. Int. J. Health Geogr. 2014;13:18. doi: 10.1186/1476-072X-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Preventive Services Task Force Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2008;149(9):627. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- Wernli K.J., Hubbard R.A., Johnson E. Patterns of colorectal cancer screening uptake in newly eligible men and women. Cancer Epidemiol. Biomark. Prev. 2014;23(7):1230–1237. doi: 10.1158/1055-9965.EPI-13-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler S.B., Carpenter W.R., Peppercorn J., Schenck A.P., Weinberger M., Biddle A.K. Structural/organizational characteristics of health services partly explain racial variation in timeliness of radiation therapy among elderly breast cancer patients. Breast Cancer Res. Treat. 2012;133(1):333–345. doi: 10.1007/s10549-012-1955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler S.B., Kuo T.M., Goyal R.K. Regional variation in colorectal cancer testing and geographic availability of care in a publicly insured population. Health Place. 2014;29:114–123. doi: 10.1016/j.healthplace.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Town M., Balluz L.S. Surveillance for certain health behaviors among states and selected local areas - United States, 2010. MMWR Surveill. Summ. 2013;62(1):1–247. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Colorectal cancer testing modality description and billing codes.