Abstract
The idiopathic inflammatory myopathies (IIM) are a heterogeneous group of rare autoimmune diseases characterized by muscle weakness and extramuscular manifestations such as skin rashes and interstitial lung disease. We genotyped 2,566 IIM cases of Caucasian descent using the Immunochip; a custom array covering 186 established autoimmune susceptibility loci. The cohort was predominantly comprised of dermatomyositis (DM, n=879), juvenile dermatomyositis (JDM, n=481), polymyositis (PM, n=931) and inclusion body myositis (IBM, n=252) patients collected from 14 countries through the Myositis Genetics Consortium. The human leukocyte antigen (HLA) and PTPN22 regions reached genome-wide significance (p<5×10−8). Nine regions were associated at a significance level of p<2.25×10−5, including UBE2L3, CD28 and TRAF6, with evidence of independent effects within STAT4. Analysis of clinical subgroups revealed distinct differences between PM, and DM and JDM. PTPN22 was associated at genome-wide significance with PM, but not DM and JDM, suggesting this effect is driven by PM. Additional suggestive associations including IL18R1 and RGS1 in PM and GSDMB in DM were identified. HLA imputation confirmed that alleles HLA-DRB1*03:01 and HLA-B*08:01 of the 8.1 ancestral haplotype (8.1AH) are most strongly associated with IIM, and provides evidence that amino acids within the HLA, such as HLA-DQB1 position 57 in DM, may explain part of the risk in this locus. Associations with alleles outside the 8.1AH reveal differences between PM, DM, and JDM. This work represents the largest IIM genetic study to date, reveals new insights into the genetic architecture of these rare diseases and suggests different predominating pathophysiology in different clinical subgroups.
Keywords: Genetics, idiopathic inflammatory myopathy, myositis, HLA, association
Introduction
The idiopathic inflammatory myopathies (IIM) are a heterogeneous group of rare autoimmune diseases, the major phenotypes of which are dermatomyositis (DM), polymyositis (PM), inclusion body myositis (IBM) and DM/PM overlapping with other connective tissue diseases.[1] IIM are primarily characterised by the presence of proximal muscle weakness, elevated levels of skeletal muscle enzymes and inflammatory infiltrates in skeletal muscle, but may also present with extramuscular manifestations including skin rashes, interstitial lung disease and malignancy that often correlate with serum antibody status.[2]
IIM are thought to be complex genetic diseases, initiated by immune activation following specific environmental events in genetically predisposed individuals. The strongest genetic association in the IIM has been consistently within the major histocompatibility complex (MHC),[3] specifically with the 8.1 ancestral haplotype (8.1 AH). A recent genome-wide association study (GWAS) in DM, and a candidate gene study, also indicate overlap of genes implicated in other autoimmune diseases.[4, 5] The Immunochip is a custom-designed array containing coverage of 186 established autoimmune susceptibility loci and extended coverage across the MHC.[6] In this study, we report an Immunochip analysis of 2,566 global IIM cases and 15,651 controls, representing the largest genetic association study to date in IIM.
Methods
Samples
2,954 samples from 14 countries were collected through the Myositis Genetics Consortium (MYOGEN), and written informed consent was obtained from all cases with approval from research ethics boards at each participating centre. There is overlap between these samples and previous IIM genetic studies.[3–5] IIM cases were included if they fulfilled probable or definite Bohan and Peter classification criteria for PM, juvenile PM (JPM), DM or Juvenile DM (JDM),[7, 8] and Griggs or European Neuromuscular Centre (ENMC) or Medical Research Council (MRC) criteria for IBM.[9–11] Eleven samples met the criteria for anti-synthetase syndrome,[12] however available clinical data was not able to differentiate between PM or DM. These were included in the combined IIM analysis, but removed from the clinical subgroup analysis.
Immunochip control data from 12 countries was provided by four disease consortia (online supplementary methods).
Genotyping and quality control
Genotyping was performed in accordance with Illumina’s protocols in the UK (Centre for Genetics and Genomics Arthritis Research UK, University of Manchester, UK) and the US (Feinstein Institute, New York, USA). Standard sample and SNP QC was performed in PLINK v1.07 (online supplementary methods).
Statistical Analysis
Statistical analysis was performed in PLINK v1.07 using a logistic regression applying an additive model, including the top ten principal components as covariates to control for population stratification. Evidence for additional independent effects was investigated using a stepwise logistic regression including the most associated variants as covariates in subsequent conditional analyses.
Functional annotation
Associated loci were interrogated for potentially causative variants using eQTL databases, and the functional prediction tools PolyPhen-2,[13] SIFT,[14] and phastCons17-way[15] (online supplementary methods).
MHC imputation and association analysis
Classical human leukocyte antigen (HLA) alleles and corresponding amino acid sequences were imputed using SNP2HLA. A logistic regression assuming an additive model was used to test for association, and forward stepwise logistic regression was used to test for independent effects (online supplementary methods). Classical 4-digit HLA alleles were preferentially reported, unless an amino acid association explained more risk than HLA alleles alone.
Results
Genotyping quality control
After stringent SNP and sample quality control we analysed 90,536 genetic variants in 2,566 IIM cases and 15,651 controls of Caucasian descent (Table 1). A breakdown of this cohort by clinical subgroup is reported in Table S1. Australia, Denmark and Switzerland did not have an ethnically matched control group; however, these were adequately matched by existing cohorts (UK, Sweden, and Germany respectively). By including the top ten principal components as covariates and calculating the genomic inflation on a set of null SNPs (from a study investigating the genetic basis for reading and writing ability)[16] on the Immunochip gave a λGC1000 = 1.05, indicating that cases and controls are well matched for ethnicity (online supplementary Figure S1).
Table 1.
Number of IIM cases included in the analysis.
| Number of Cases | Number of Controls | ||
|---|---|---|---|
| Australia | 120 | - | |
| Belgium | 12 | Belgium | 351 |
| Czech Republic | 236 | Poland | 526 |
| Denmark | 53 | - | |
| France | 37 | France | 497 |
| Hungary | 209 | Hungary Germany |
257 1029 |
| Italy | 37 | Italy Italy (RAF cohort) |
969 813 |
| Netherlands | 38 | Netherlands | 2020 |
| Norway | 63 | Norway | 730 |
| Sweden | 269 | Sweden | 1938 |
| Spain | 73 | Spain | 409 |
| Switzerland | 3 | - | |
| United Kingdom | 993 | United Kingdom | 4332 |
| United States | 423 | United States | 1780 |
| Total | 2,566 | Total | 15,651 |
Number of IIM cases after QC, by country of origin. Control samples shared from Immunochip consortia matched by closest ethnicity.
HLA and PTPN22 are the most strongly associated regions in IIM
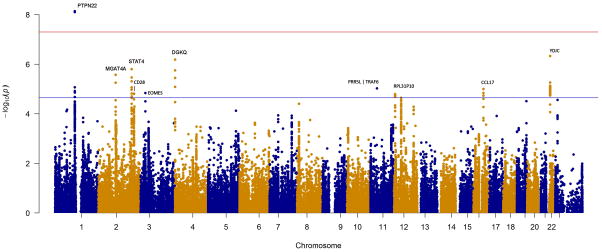
Two regions in this study reached genome-wide significance (p<5×10−8) (Figure 1A and Table 2). As expected, the most strongly associated region was within the MHC (p=9×10−133) (online supplementary Figure S2). HLA-imputation was performed separately on this locus.
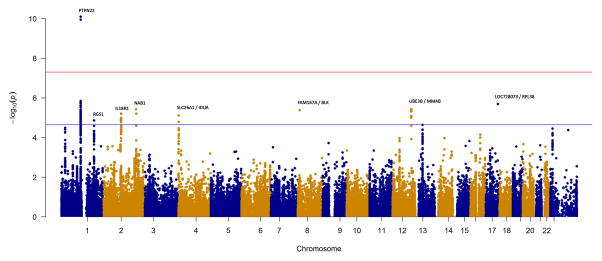
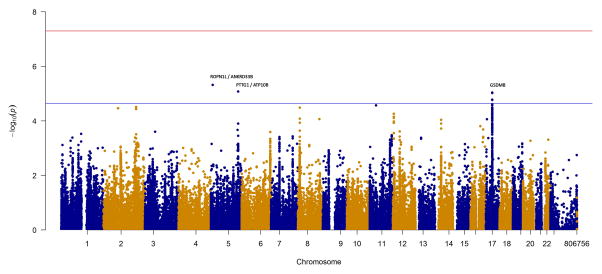
Figure 1. Manhattan plots of the IIM, PM and DM+JDM analyses, with the MHC region removed.
The red and blue lines represent genome-wide level of significance (p=5×10−8) and suggestive significance (p=2.25×10−5) respectively. A) Analysis of 2,566 IIM cases and 15,651 controls. B) Analysis of 931PM cases and 15,651 controls. C) Analysis of 1,360 DM+JDM cases and 15,651 controls.
Table 2.
Loci associated with IIM, PM, DM and JDM cases
| Subgroup | Gene Region | Chr | Position | Most Significant SNP | Minor Allele | MAF cases | MAF controls | P-value | OR (95% CI) | Localisation of LD to nearest genes (r2 ≥ 0.9) | Overlap with other autoimmune diseases |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IIM | HLA | 6 | 32395726 | rs3129843 | G | 0.24 | 0.11 | 9.14×10−133 | 2.74 (2.53–2.97) | MHC | Multiple |
| IIM | PTPN22 | 1 | 114377568 | rs2476601 | A | 0.12 | 0.09 | 7.22×10−9 | 1.32 (1.20–1.45) | Exon 12 of PTPN22 | ATD,[17] IBD,[16] JIA,[18] RA,[19] SLE,[20] SSC,[21] T1D,[22] VIT,[23] |
| IIM | YDJC | 22 | 21985094 | rs5754467 | G | 0.23 | 0.20 | 4.67×10−7 | 1.21 (1.12–1.30) | Intron 1 of UBE2L3 | complete YDJC | CEL,[6] IBD,[16] JIA,[18] PSO,[24] RA,[19] SLE,[20] |
| IIM | DGKQ | 4 | 956047 | rs6599390 | A | 0.30 | 0.34 | 6.48×10−7 | 0.85 (0.79–0.90) | Intron 21 of DGKQ to intron 2 of SLC26A1 and intron 2 of IDUA | |
| IIM | STAT4 | 2 | 191917317 | rs4853540 | T | 0.19 | 0.22 | 1.57×10−6 | 0.83 (0.77–0.89) | Intron 3 to intron 14 of STAT4 | JIA,[18] PBC,[25] RA,[19] SLE,[20] |
| IIM | MGAT4A | 2 | 99389870 | rs10189330 | T | 0.50 | 0.46 | 2.68×10−6 | 1.16 (1.09–1.23) | Intergenic of C2orf55 (KIAA1211L) and MGAT4A incorporating uncharacterized LOC101927070 | |
| IIM | PRR5L | TRAF6 | 11 | 36492191 | rs570676 | A | 0.37 | 0.40 | 9.42×10−6 | 0.87 (0.82–0.92) | Intergenic PRR5L (also known as FLJ14213) | complete TRAF6 | |
| IIM | CCL17 | 16 | 57445376 | rs223900 | T | 0.28 | 0.25 | 9.97×10−6 | 1.17 (1.09–1.25) | Intron 1 of CCL17 | |
| IIM | EOMES | 3 | 28076283 | rs376072 | T | 0.26 | 0.29 | 1.45×10−5 | 0.86 (0.80–0.92) | 310.68kb upstream of EOMES | |
| IIM | CD28 | 2 | 204592021 | rs3116494 | G | 0.28 | 0.26 | 1.54×10−5 | 1.16 (1.09–1.24) | Intron 1 to 8.5kb downstream of CD28 | PSC,[26] RA,[19] |
| IIM | RPL31P10 | 12 | 6523249 | rs11064180 | T | 0.38 | 0.41 | 1.61×10−5 | 0.87 (0.82–0.93) | Intergenic of LTBR and CD27 | |
| PM | HLA | 6 | 31434366 | rs3094013 | T | 0.25 | 0.12 | 6.36×10−76 | 2.97 (2.64–3.33) | MHC | Multiple |
| PM | PTPN22 | 1 | 114377568 | rs2476601 | A | 0.12 | 0.09 | 7.90×10−11 | 1.58 (1.38–1.81) | Exon 12 of PTPN22 | ATD,[17] IBD,[16] JIA[18], RA,[19] SLE,[20] SSC,[21] T1D,[22] VIT,[23] |
| PM | LOC728073 | RPL38 | 17 | 71527243 | rs9905921 | C | 0.48 | 0.43 | 2.01×10−6 | 1.26 (1.14–1.39) | Intronic (provisional in refseq) of SDK2 | |
| PM | UBE3B | MMAB | 12 | 109980516 | rs7956536 | C | 0.44 | 0.46 | 3.66×10−6 | 0.80 (0.72–0.88) | Intron 19 of MYO1H, complete KCTD10; UBE3B; MMAB; MVK | |
| PM | NAB1 | 2 | 191535576 | rs2286896 | G | 0.15 | 0.13 | 3.76×10−6 | 1.35 (1.19–1.53) | Complete NAB1 | |
| PM | FAM167A | BLK | 8 | 11333521 | rs17799348 | T | 0.36 | 0.39 | 4.13×10−6 | 0.80 (0.71–0.87) | Intergenic FAM167A | BLK | |
| PM | IL18R1 | 2 | 103012902 | rs1420095 | G | 0.07 | 0.09 | 6.16×10−6 | 0.63 (0.52–0.78) | Intron 3 of IL18R1 : 1L18RAP | |
| PM | SLC26A1 | IDUA | 4 | 980464 | rs4690220 | G | 0.49 | 0.45 | 7.47×10−6 | 1.25 (1.13–1.37) | 2.14 Kb 3’ to intron 2 of SLC26A1 | SJO,[27] |
| PM | RGS1 | 1 | 192545099 | rs7535818 | G | 0.17 | 0.18 | 1.37×10−5 | 0.74 (0.65–0.85) | 25kb upstream to intron 1 of RGS1 | CEL,[6] MS,[28] T1D,[22] |
| DM+JDM | HLA | 6 | 32395726 | rs3129843 | G | 0.21 | 0.11 | 1.72×10−48 | 2.18 (1.97–2.42) | MHC | Multiple |
| DM+JDM | ROPN1L | ANKRD33B | 5 | 10517908 | rs4702698 | G | 0.34 | 0.30 | 4.77×10−6 | 1.22 (1.12–1.33) | Intergenic of ROPN1L and ANKRD33B | |
| DM+JDM | PTTG1 | ATP10B | 5 | 159928876 | rs4921293 | G | 0.39 | 0.35 | 8.27×10−6 | 1.21 (1.11–1.31) | Intergenic of PTTG1 and ATP10B | |
| DM+JDM | GSDMB | 17 | 38066267 | rs1008723 | T | 0.47 | 0.49 | 9.05×10−6 | 1.20 (1.11–1.30) | Complete IKZF3, ZPBP2 GSDMB | IBD,[16] MS,[28] PBC,[25] RA,[29] T1D,[30] |
Analysis of 2,566 IIM cases, 931 PM cases, and 1,360 DM and JDM cases, compared to 15,651 controls. Loci reported at genome wide significance p <5×10−8 (bold) or at suggestive significance p <2.25×10−5. Coordinates based on GRCh37 assembly. Overlap defined as high LD (r2 >0.7) between the associations in IIM (including independent effects) and the lead association in another autoimmune disease (as reported on www.immunobase.org). ATD – Autoimmune Thyroid Disease, CEL – Celiac Disease, Chr – chromosome, IBD – Inflammatory Bowel Disease, JIA – Juvenile Idiopathic Arthritis, MAF – minor allele frequency, MS – Multiple Sclerosis, OR – Odds ratio, PBC – Primary Biliary Cirrhosis, PSC - Primary Sclerosing Cholangitis, PSO – Psoriasis, RA – Rheumatoid Arthritis, SJO - Sjogren Syndrome, SLE – Systemic Lupus Erythematosus, SSC - Systemic Scleroderma, T1D – Type 1 Diabetes, VIT – Vitiligo, 95% CI – 95% confidence interval
The other region reaching genome-wide significance was within the PTPN22 locus (rs2476601; p=7.22×10−9), an established autoimmune risk locus. This SNP/locus has been previously associated in an IIM candidate gene study,[3] but was not associated in a GWAS in DM.[4]
A further nine regions were associated at a suggestive level of significance
We next investigated associations reaching our suggestive tier of association (p=2.25×10−5) calculated using the genetic Type 1 Error Calculator.[31] This estimates the effective number of independent tests based on the LD between SNPs contained on the genotyping array. Here, we found evidence of a further nine associated loci (Table 2).
The third most strongly associated SNP in this analysis was in the YDJC gene (rs5754467) on chromosome 22 (4.67×10−7). This SNP tags a large haplotype block containing UBE2L3, an established autoimmune risk locus.
STAT4 is a susceptibility locus for many autoimmune diseases. The lead SNP in this region was protective, which is a novel finding in IIM (rs4853540, p=1.57×10−6). Stepwise logistic regression analysis in this region suggested an independent risk effect of rs10174238 (p=1.08×10−5, OR=1.17, 95% CI=1.09–1.26) (online supplementary Figure S3) and a further potential independent effect was seen at rs932169 (p=2.88×10−5, OR=1.25, 95% CI=1.13–1.39).
Further variants reaching our suggestive significance threshold reveal loci of interest that have been previously associated with autoimmune disease, including DGKQ, EOMES, CD28 and PRR5L/TRAF6. Associated SNPs that tag risk haplotypes (r2 >0.7) in other autoimmune diseases are reported in Table 2, and the direction of effect is reported in online supplementary Table S2.
Subgroup analysis reveals unique associations within PM and DM
We stratified our cohort by the two largest subgroups within IIM, consisting of 931 adult PM cases (Figure 1B, Table 2) and 1,360 DM cases (Figure 1C, Table 2). JDM cases were included in the DM analysis both to increase power, and on previous evidence that there is not extensive genetic heterogeneity between the subgroups.[4] The only non-HLA region to reach genome-wide significance in either subgroup was PTPN22 in PM (rs2476601, p=7.9×10−11). Interestingly, with a smaller sample size, the association in PM with PTPN22 was stronger than in the combined IIM analysis. There was no evidence of association in DM (p=0.19), therefore the stronger effect in PM appears to be driving the association in the combined IIM analysis. Other interesting regions reaching a suggestive level of significance were SL26A1/IDUA and RGS1 in PM, and GSDMB in DM (online supplementary Table S3).
Exonic and eQTL SNPs suggest potential causal variants
Potential functionally relevant variants were investigated for non-synonymous SNPs (Table 3) or eQTLs (Table 4) that are tagged by the lead SNP (r2 > 0.9). Two variants within the GSDMB gene, suggestively associated in DM, are ‘potentially damaging’ as predicted by PolyPhen-2. The PTPN22 variant is confirmed to be conserved across vertebrates, as well as a SNP in UBE3B. Evidence for eQTLs in cells with immune function (lymphoblastoid cell lines and monocytes) was found in six loci and may help annotate our associations, for example, the association with NAB1 in PM may be due to an eQTL affecting the expression of STAT1, 275Kb upstream.
Table 3.
Potentially causal exonic SNPs.
| Subgroup | Mapped Genes | Most Significant SNP | SNP in LD | SNP Location | Amino acid change | PolyPhen-2/SIFT | phastCons17-way |
|---|---|---|---|---|---|---|---|
| IIM + PM | PTPN22 | rs2476601 | rs2476601 | Non-synonymous coding in PTPN22 | R620W | Benign/tolerated | 0.99 |
|
| |||||||
| IIM | DGKQ | rs6599390 | rs3796622 | Non-synonymous coding SLC26A1 | Q556R | Benign/tolerated | 0.35 |
|
| |||||||
| PM | UBE3B | MMAB | rs7956536 | rs7298565 | Non-synonymous coding UBE3B | R346Q | Benign/tolerated | 1 |
|
| |||||||
| rs9593 | Non-synonymous coding in MMAB | M239K | Benign/tolerated | 0.17 | |||
|
| |||||||
| DM+JDM | GSDMB | rs1008723 | rs11078928 | Splice acceptor variant in GSDMB | 0 | ||
| rs2305480 | Non-synonymous coding GSDMB | P298S | Possibly damaging/Tolerated | 0 | |||
| rs2305479 | Non-synonymous coding GSDMB | G291R | Probably damaging/Tolerated | 0 | |||
SNPs investigated for functionality may be the lead association or any SNP with r2 ≥0.9. PolyPhen-2,[13] and SIFT,[14] was used to predict the possible impact of an amino acid substitution. phastCons17-way,[15] gives a score of evolutionary conservation in 17 vertebrates, with 1 being the most conserved at that base position.
Table 4.
Evidence of expression quantitative trait loci (eQTL)
| Subgroup | Mapped Genes | Immunochip lead SNP | eQTL within r2 >0.9 | References |
|---|---|---|---|---|
| IIM | UBE2L3 | YDJC | rs11089637 | UBE2L3, RIMBP3 | [32] [33] |
| PM | UBE3B | MMAB | rs7956536 | KCTD10, MMAB | [32] [33] [34] |
| PM | RGS1 | rs7535818 | RGS1 | [32] |
| PM | NAB1 | rs2286896 | STAT1 | [35] |
| DM+JDM | GSDMB | rs1008723 | ORMDL3, MED24, KRT222, NR1D1, GSDMB | [34] [35] [36] [33] |
eQTLs for the expression of a gene in cells with an immune function (Lymphoblastoid and Monocytes). RegulomeDB,[37] Genevar,[38] and the Pritchard lab eQTL browser (http://eqtl.uchicago.edu/cgi-bin/gbrowse/eqtl/) were interrogated. The most significant SNP or any SNP with r2 ≥0.9 with most significant SNP was used. Genes are indicated along with studies in which the eQTLs were reported.
HLA Imputation confirms alleles of the 8.1 ancestral haplotype as the strongest association in IIM
Due to the complex linkage disequilibrium/haplotype structure in the MHC, interpretation of causal associations and independent effects using SNPs may be inadequate. We used SNP2HLA to impute classical HLA alleles and amino acids from SNP genotyping information. For each analysis, all variants reaching statistical significance (p<6.8×10−6) after each round of conditioning are included in online supplementary Table S4–S15. For many associations, amino acids unique to classical HLA risk alleles were associated at similar levels of significance to the HLA allele. For consistency, 4-digit HLA alleles are reported, unless an amino acid is significantly more associated than individual HLA alleles. In the combined IIM analysis (n=2,566), the most associated variants were classical HLA alleles, with HLA-DRB1*03:01 being the most significant 4-digit allele (p=2.58×10−135, OR=1.88, 95% CI=1.68–2.11). HLA-DRB1*03:01 forms part of the 8.1 AH which has been consistently associated with IIM. After conditioning on the effects of HLA-DRB1*03:01, a strong association was found with HLA-B*08:01 (p=3.23×10−14, OR=1.58, 95% CI=1.41–1.78) suggesting that there is an independent effect within this locus. Further residual associations highlight the heterogeneity within this cohort, so analysis was then conducted on clinical subgroups. Similar associations were found with PM (n=931), HLA-DRB1*03:01 being the most significant 4-digit allele (p=6.11×10−80, OR=1.99, 95% CI=1.67–2.36) and an independent effect with HLA-B*08:01 (p=4.17×10−9, OR=1.71, 95% CI=1.43–2.05). As the effect size of the HLA is strong in IIM, we hypothesised that we may be able to detect any potential differences between adult and juvenile DM, even with a reduced sample size. In adult DM (n=879), HLA-B*08:01 was the most significant allele (p=2.46×10−42, OR=1.90, 95% CI=1.66–2.17). Conditioning on HLA-B*08:01, there was evidence of multiple independent effects within the HLA-DQB1 locus, therefore we analysed imputed amino acid residues. Amino acid position 57 of HLA-DQB1 was more significantly associated with DM than individual HLA-DQB1 alleles (p=8.95×10−14), with alanine (p=1.29×10−12 OR=1.62, 95% CI=1.44–1.83) and serine (p=9.28×10−7, OR=2.15, 95% CI=1.60–2.84) conferring the greatest risk. Further association with HLA-DQB1 remains after conditioning, notably an independent effect of HLA-DQB1*04:02 (p=2.01×10−6, OR=1.99, 95% CI=1.52–2.58). In the JDM subgroup (n=481), HLA-DRB1*03:01 was the most associated allele (p=7.91×1014, OR=1.90, 95% CI=1.61–2.22) and an independent association was observed with HLA-C*02:02 (p=3.28×10−7, OR=1.99, 95% CI=1.55–2.52) which is not a part of the 8.1 ancestral haplotype.
Discussion
This is the largest genetic study to date in IIM, and has revealed several novel suggestive associations in adult and juvenile IIM emphasising the autoimmune architecture of these diseases. We have confirmed HLA and PTPN22 as the most strongly associated regions in IIM, and identified nine additional associations at a suggestive level of significance. Subgroup analysis has identified distinct differences between PM and DM. Identification of exonic and eQTL SNPs has localised association signals to several potential causal variants.
It is reassuring that associations such as PTPN22, STAT4 and UBE2L3 follow a similar genetic profile as reported in other autoimmune diseases. The most significantly associated SNP in the PTPN22 region is the rs2476601 variant, a C>T polymorphism that results in a non-synonymous arginine (R) to tryptophan (W) amino acid change at position 620. Although this SNP has been extensively studied in the context of autoimmunity, there is no consensus regarding the functional consequences of this SNP. Some studies report a gain of function mutation by enhancing the inhibitory effect on TCR signalling,[39] while others report a loss of function by increased degradation of the protein and a diminished inhibitory effect on T-cell activation.[40]
STAT4 is an important transcription factor for many genes involved in T-cell differentiation and has previously been associated with DM in the Japanese population.[41] Stepwise logistic regression analysis was conducted on all regions in this study; however STAT4 is the only locus with evidence of independent associations. The three independent SNPs are in LD with associations in different diseases. The lead SNP in STAT4 is protective, and in moderate LD with protective SNPs in STAT4 reported in inflammatory bowel disease (IBD), Crohn’s and ulcerative colitis.[16] The independent risk effect of rs10174238 is the same SNP reported in juvenile idiopathic arthritis,[18] and is in strong LD with disease-associated SNPs in RA and SLE.[19, 42] A SNP in high LD with rs932169 has been reported to be associated with primary biliary cirrhosis.[25]
The most significantly associated SNP in the YDJC gene tags an established autoimmune susceptibility locus where UBE2L3 is thought to be the causal gene.[43] This risk haplotype is thought to increase UBE2L3 expression in B cells and monocytes and amplify NF-κB activation.[43]
Stratification by clinical subgroup revealed further novel suggestive associations. These distinct differences between PM and DM suggest different autoimmune pathways in these subsets of IIM. For example, when splitting the total IIM cohort into PM and DM, we have shown that the association with PTPN22 is predominantly driven by a strong association with PM. For all associations, we have stratified by clinical subgroup and reported the summary statistics in online supplementary Table S3. IBM patients were included in the combined IIM analysis on the basis of their diagnosis as an inflammatory autoimmune myopathy, however we did not analyse this subgroup separately due to a lack of power (n=252). Removing this group from our analyses did not make any substantial difference in associated regions, however the strength of the signals were attenuated in most instances. With eight non-HLA loci reaching our level of suggestive significance in PM, and only 3 in DM, it may be that the Immunochip is explaining less of the genetic risk to DM. This may be due to lack of power, the selected content of the Immunochip, heterogeneity of phenotypes within DM, or a weaker genetic influence compared to other autoimmune diseases. Some previous reported associations with DM failed to replicate in this study. We looked for evidence of association with loci that have previously been associated with IIM that did not reach our suggestive level of significance. For example, in the DM and JDM subgroup analysis, an association was found with rs2618476 (p=3.2×10−5, OR=1.2, 95% CI=1.1–1.32), a SNP in B Lymphoid Tyrosine Kinase (BLK). rs2618476 is a proxy for a SNP that was associated with DM in the Japanese population,[44] and is also highly correlated (r2>0.8) with associations found in SLE and RA.[20, 29] With this knowledge, this association becomes more convincing, whereas the single association in the FAM167A-BLK region in the PM subgroup (rs17799348) that is independent of the established risk haplotype, is less so.
It is important to note that this study was conducted on Caucasian IIM individuals. While there is evidence that risk loci may be shared across populations, such as STAT4 and BLK in the Japanese population, the association between PTPN22 and autoimmune disease is unique to Caucasians as the R620W variant is rarely seen in Asian populations. There is therefore a need to conduct further genetic studies on different IIM populations.
The Immunochip contains a dense set of SNPs covering 186 loci based on evidence of association with 12 different autoimmune and inflammatory diseases.[18] IIM was not one of these diseases, so this study can be seen as an exploratory investigation to assess genetic overlap with other autoimmune diseases, rather than the identification of genes novel to IIM. With 2,566 samples, Immunochip studies of similar size have revealed multiple non-HLA associations reaching genome-wide significance.[18, 45] The fact that only a single locus reached this threshold may due to low statistical power owing to phenotypic heterogeneity within IIM. A more conservative level of significance (p<2.25×10−5) revealed suggestive associations of interest. SNPs that are the same, or in high LD with established autoimmune variants, along with biological knowledge and/or evidence of functionality may lead us to pursue these associations with more confidence. Indeed, a recent Immunochip study in T1D calculated a Bayesian posterior probability of disease association >0.9 of SNPs reaching a suggestive level of significance (p<1×10−5) when there is evidence of genome-wide significance in other Immunochip studies.[30]
Due to the extended haplotypes that are present in the HLA region, for many associations, alleles carried on the same haplotype reached an equivalent significance level. For consistency, the most associated allele was used in the stepwise conditional analysis; however, this is not to say that the allele is causative. Interestingly in PM, two alleles frequently inherited together on the 8.1 AH (HLA-DRB1*03:01 and HLA-B*08:01) show evidence of having independent effects. This may also be the case in DM, however, after conditioning on HLA-B*08:01, the association with other alleles of the 8.1 AH did not reach genome-wide significance. In DM, the independent association with amino acid position 57 in HLA-DQB1 may explain part of the risk within this gene. Indeed, this position is an established risk factor for T1D.[46] In this study, classical 4-digit HLA alleles were preferentially reported, unless an amino acid association explained more risk than HLA alleles alone. However looking at amino acids may give insight into functionality. For example, the association with HLA-DRB1*03:01 may be explained by the presence of amino acids that are unique to that allele. An asparagine at position 77, and an arginine at position 74 also were highly associated with IIM (online supplementary Table S4), and these residues are predominantly found on DRB1*03 alleles. As there are multiple residues unique to this allele, it is hard to tease out which positions may be functionally important; however the location of these amino acids in the HLA-DRB1 molecule may give insight (online supplementary Figure S4). Amino acid position 74 of HLA-DRB1 lies within the peptide binding groove, and almost all of the risk at this position can be explained by the presence of an arginine (p=3.1×10−72, OR=2.83, 95% CI=2.53–3.17). The location of Arg-74 may change the structure of the peptide binding groove in such a way as to accommodate autoantigenic peptides. Indeed Arg-74 is an established risk factor for the development of autoimmune diseases.[47] A similar phenomenon is seen with HLA-B*08:01 and the occurrence of Phe-67 and Asp-9 (online supplementary Figure S5).
Alleles of the 8.1 AH have frequently been associated with the presence of myositis autoantibodies such as anti-Jo-1 and anti-PM-Scl. It may be that the association with the 8.1 AH and IIM is due to the prevalence of these antibodies, and weak associations with other HLA-alleles may be due to associations with autoantibodies that are less frequent in the disease subgroup. Further work is planned to stratify patients by serotype to clarify these differences.
This study has revealed new suggestive associations with IIM in the Caucasian population, and independent associations with PM and DM. and has shown that subgrouping patients into clinical subgroups is important to expand our knowledge of IIM. This international collaboration has made it possible to perform the largest study to date in IIM and it has considerably expanded our knowledge about the genetic architecture of this rare disease.
Supplementary Material
Acknowledgments
We thank Mrs. Fiona Marriage (Centre for Integrated Genomic Medical Research, University of Manchester) for technical support, Drs. Elaine Remmers, Douglas Bell (National Institutes of Health) and Jonathan Massey (University of Manchester) for critical review of the manuscript, and Mr. Paul New (Salford Royal Foundation Trust) for ethical and technical support. We thank all of the patients and their families who contributed to this study.
This publication was supported by researchers at the National Institute for Health Research (NIHR) Biomedical Research Unit Funding Scheme and the University College London Hospitals Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the (UK) National Health Service (NHS), the NIHR or the (UK) Department of Health.
Further MYOGEN Investigators
Study investigators of the Myositis Genetics Consortium, in addition to the authors of this article, are as follows: Drs. Christopher Denton (Royal Free Hospital, London, UK), Herman Mann (Institute of Rheumatology, Prague), David Hilton-Jones (John Radcliffe Hospital, Oxford, UK), Patrick Kiely (St. George’s Hospital, London, UK), Paul H. Plotz, Mark Gourley (National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD), Kelly Rouster-Stevens (Emory University School of Medicine, Atlanta, GA), Adam M Huber (Dalhousie University, Halifax, Nova Scotia, Canada), Galina Marder (North Shore Univeristy Hospital, Great Neck, NY) and Mazen Dimachkie (University of Kansas Medical Center, Kansas City, KS).
Funding
This study was supported in part by: Association Francaise Contre Les Myopathies (AFM), The European Union Sixth Framework Programme (project AutoCure; LSH-018661), European Science Foundation (ESF) in the framework of the Research Networking Programme European Myositis Network (EUMYONET), The Swedish Research Council and The regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the intramural research program of the National Institute of Environmental Health Sciences (NIEHS), the National Institutes of Health (NIH); European Community’s FP6, AutoCure LSHB CT-2006-018661; The UK Myositis Support Group; Arthritis Research UK (18474); The Cure JM Foundation; the European Science Foundation; the Wellcome Trust; the Henry Smith Charity UK; Action Medical UK; and the Swedish Research Council. The Czech cohort was supported by Project for Conceptual Development of Research Organization 00023728 from Ministry of Health in the Czech Republic.
Shared Immunochip Control Cohorts
We thank the Rheumatoid Arthritis Consortium International (RACI) for Netherlands, Spanish, Swedish, UK and US Immunochip control genotypes. This study makes use of data generated by the Wellcome Trust Case-Control Consortium. A full list of the investigators who contributed to the generation of the data is available from www.wtccc.org.uk. Funding for the project was provided by the Wellcome Trust under award 076113 and 085475. Swedish control data was provided from EIRA study, Professor Lars Alfredsson, Department of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden. Control data from the Netherlands was provided from the department of Rheumatology, Leiden University Medical Center, Leiden, The Netherlands.
Polish control data was provided by the Celiac Disease Consortium and Hungarian control data collected with the help of the Hungarian Research Fund (OTKA) grant K101788 was provided by Prof Ilma. Korponay-Szabo, Celiac Disease Centre, Heim Pál Children’s Hospital, Budapest and University of Debrecen, Debrecen Hungary.
We acknowledge the International MS Genetics Consortium for providing access to control sample data from Belgium, France, Norway, Italy and Germany. The collection and genotyping of these samples was made possible by: the Norwegian MS society and the Norwegian Bone Marrow Registry (Norwegian samples); the French Biological Resource Center for MS Genetics, Genethon and INSERM (French samples); and a FISM (Italian Foundation for Multiple Sclerosis) grant (“Progetto Speciale Immunochip”) for Italian samples.
Italian samples were collected by Prof. Sandra D’Alfonso (Interdisciplinary Research Center of Autoimmune Diseases IRCAD, University of Eastern Piedmont, Novara, Italy; PROGEMUS Consortium) and Dr. Martinelli Boneschi (Laboratory of Genetics of Complex Neurological Disorders, Division of Neuroscience & INSPE, San Raffaele Scientific Institute, Milan, Italy; PROGRESSO Consortium); funding was provided by a FISM (Italian Foundation for Multiple Sclerosis) grant (“Progetto Speciale Immunochip). The KORA study was initiated and financed by the Helmholtz Zentrum München – German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria. Furthermore, KORA research was supported within the Munich Center of Health Sciences (MC-Health), Ludwig-Maximilians-Universität, as part of LMUinnovativ.
The SweMyoNet
Maria Lidén (Uppsala University), Awat Jalal (Örebro University), Helena Hellström (Rheumatology Clinic Falun), Thomas Skogh (Linköping University), Aladdin Mohammad (Lund).
UK Adult Onset Myositis Immunogenetic Collaboration (UKMYONET)
Members of the UK Adult Onset Myositis Immunogenetic Collaboration who recruited and enrolled subjects are as follows: Drs. Yasmeen Ahmed (Llandudno General Hospital), Raymond Armstrong (Southampton General Hospital), Robert Bernstein (Manchester Royal Infirmary), Carol Black (Royal Free Hospital, London), Simon Bowman (University Hospital, Birmingham), Ian Bruce (Manchester Royal Infirmary), Robin Butler (Robert Jones & Agnes Hunt Orthopaedic Hospital, Oswestry), John Carty (Lincoln County Hospital), Chandra Chattopadhyay (Wrightington Hospital), Easwaradhas Chelliah (Wrightington Hospital), Fiona Clarke (James Cook University Hospital, Middlesborough), Peter Dawes (Staffordshire Rheumatology Centre, Stoke on Trent), Joseph Devlin (Pinderfields General Hospital, Wakefield), Christopher Edwards (Southampton General Hospital), Paul Emery (Academic Unit of Musculoskeletal Disease, Leeds), John Fordham (South Cleveland Hospital, Middlesborough), Alexander Fraser (Academic Unit of Musculoskeletal Disease, Leeds), Hill Gaston (Addenbrooke’s Hospital, Cambridge), Patrick Gordon (King’s College Hospital, London), Bridget Griffiths (Freeman Hospital, Newcastle), Harsha Gunawardena (Frenchay Hospital, Bristol), Frances Hall (Addenbrooke’s Hospital, Cambridge), Beverley Harrison (North Manchester General Hospital), Elaine Hay (Staffordshire Rheumatology Centre, Stoke on Trent), Lesley Horden (Dewsbury District General Hospital), John Isaacs (Freeman Hospital, Newcastle), Adrian Jones (Nottingham University Hospital), Sanjeet Kamath (Staffordshire Rheumatology Centre, Stoke on Trent), Thomas Kennedy (Royal Liverpool Hospital), George Kitas (Dudley Group Hospitals Trust, Birmingham), Peter Klimiuk (Royal Oldham Hospital), Sally Knights (Yeovil District Hospital, Somerset), John Lambert (Doncaster Royal Infirmary), Peter Lanyon (Queen’s Medical Centre, Nottingham), Ramasharan Laxminarayan (Queen’s Hospital, Burton Upon Trent), Bryan Lecky (Walton Neuroscience Centre, Liverpool), Raashid Luqmani (Nuffield Orthopaedic Centre, Oxford), Jeffrey Marks (Steeping Hill Hospital, Stockport), Michael Martin (St. James University Hospital, Leeds), Dennis McGonagle (Academic Unit of Musculoskeletal Disease, Leeds), Neil McHugh (Royal National Hospital for Rheumatic Diseases, Bath), Francis McKenna (Trafford General Hospital, Manchester), John McLaren (Cameron Hospital, Fife), Michael McMahon (Dumfries & Galloway Royal Infirmary, Dumfries), Euan McRorie (Western General Hospital, Edinburgh), Peter Merry (Norfolk & Norwich University Hospital, Norwich), Sarah Miles (Dewsbury & District General Hospital, Dewsbury), James Miller (Royal Victoria Hospital, Newcastle), Anne Nicholls (West Suffolk Hospital, Bury St. Edmunds), Jennifer Nixon (Countess of Chester Hospital, Chester), Voon Ong (Royal Free Hospital, London), Katherine Over (Countess of Chester Hospital, Chester), John Packham (Staffordshire Rheumatology Centre, Stoke on Trent), Nicolo Pipitone (King’s College Hospital, London), Michael Plant (South Cleveland Hospital, Middlesborough), Gillian Pountain (Hinchingbrooke Hospital, Huntington), Thomas Pullar (Ninewells Hospital, Dundee), Mark Roberts (Salford Royal Foundation Trust), Paul Sanders (Wythenshawe Hospital, Manchester), David Scott (King’s College Hospital, London), David Scott (Norfolk & Norwich University Hospital, Norwich), Michael Shadforth (Staffordshire Rheumatology Centre, Stoke on Trent), Thomas Sheeran (Cannock Chase Hospital, Cannock, Staffordshire), Arul Srinivasan (Broomfield Hospital, Chelmsford), David Swinson (Wrightington Hospital), Lee-Suan Teh (Royal Blackburn Hospital, Blackburn), Michael Webley (Stoke Manderville Hospital, Aylesbury), Brian Williams (University Hospital of Wales, Cardiff), and Jonathan Winer (Queen Elizabeth Hospital, Birmingham).
UK Juvenile Dermatomyositis Research Group
Dr. Kate Armon, Mr. Joe Ellis-Gage, Ms. Holly Roper, Ms. Vanja Briggs, and Ms. Joanna Watts (Norfolk and Norwich University Hospitals); Dr. Liza McCann, Mr. Ian Roberts, Dr. Eileen Baildam, Ms. Louise Hanna, Ms. Olivia Lloyd and Ms Susan Wadeson (The Royal Liverpool Children’s Hospital, Alder Hey, Liverpool); Dr. Phil Riley and Ms. Ann McGovern (Royal Manchester Children’s Hospital, Manchester); Dr. Clive Ryder, Mrs. Janis Scott, Mrs. Beverley Thomas, Professor Taunton Southwood, Dr. Eslam Al-Abadi and Lisa Charles (Birmingham Children’s Hospital, Birmingham); Dr. Sue Wyatt, Mrs. Gillian Jackson, Dr. Tania Amin, Dr. Mark Wood, Dr. Vanessa Van Rooyen, and Ms. Deborah Burton (Leeds General Infirmary, Leeds); Dr. Joyce Davidson, Dr. Janet Gardner-Medwin, Dr. Neil Martin, Ms. Sue Ferguson, Ms. Liz Waxman, and Mr. Michael Browne (The Royal Hospital for Sick Children, Yorkhill, Glasgow); Dr. Mark Friswell, Professor Helen Foster, Mrs. Alison Swift, Dr. Sharmila Jandial, Ms. Vicky Stevenson, Ms. Debbie Wade, Dr. Ethan Sen, Dr. Eve Smith, Ms. Lisa Qiao, Mr. Stuart Watson and Claire Duong (Great North Children’s Hospital, Newcastle); Dr. Helen Venning, Dr. Rangaraj Satyapal, Mrs. Elizabeth Stretton, Ms. Mary Jordan, Dr. Ellen Mosley, Ms. Anna Frost, Ms. Lindsay Crate, Dr. Kishore Warrier and Ms Stefanie Stafford (Queens Medical Centre, Nottingham); Professor Lucy Wedderburn, Dr. Clarissa Pilkington, Dr. N. Hasson, Mrs. Sue Maillard, Ms. Elizabeth Halkon, Ms. Virginia Brown, Ms. Audrey Juggins, Dr. Sally Smith, Mrs. Sian Lunt, Ms. Elli Enayat, Mrs. Hemlata Varsani, Miss Laura Kassoumeri, Miss Laura Beard, Miss Katie Arnold, Mrs. Yvonne Glackin, Miss Stephanie Simou, Dr. Beverley Almeida, Dr Raquel Marques, Dr Claire Deakin and Ms Stefanie Dowle (Great Ormond Street Hospital, London); Dr. Kevin Murray (Princess Margaret Hospital, Perth, Western Australia); Dr. John Ioannou and Ms. Linda Suffield (University College London Hospital); Dr. Muthana Al-Obaidi, Ms. Helen Lee, Ms. Sam Leach, Ms. Helen Smith, Dr Anne-Marie McMahon, Ms Heather Chisem and Ruth Kingshott (Sheffield’s Children’s Hospital); Dr. Nick Wilkinson, Ms. Emma Inness, Ms. Eunice Kendall, Mr. David Mayers, Ruth Etherton and Dr Kathryn Bailey (Oxford University Hospitals); Dr Jacqui Clinch, Ms Natalie Fineman and Ms Helen Pluess-Hall (Bristol Royal Hospital for Children, Bristol); Ms Lindsay Vallance (Royal Aberdeen Children’s Hospital); Ms Louise Akeroyd (Bradford Teaching Hospitals).
US Childhood Myositis Heterogeneity Study Group
The following members of the US Childhood Myositis Heterogeneity Study Group contributed to this study: Drs. Barbara S. Adams (University of Michigan, Ann Arbor, MI), Catherine A. Bingham (Hershey Medical Center, Hershey, PA), Gail D. Cawkwell (All Children’s Hospital, St. Petersburg, FL), Terri H. Finkel (Children’s Hospital of Philadelphia, Philadelphia, PA), Steven W. George (Ellicott City, MD), Harry L. Gewanter (Richmond, VA), Ellen A. Goldmuntz (Children’s National Medical Center, Washington, DC), Donald P. Goldsmith (St. Christopher’s Hospital for Children, Philadelphia, PA), Michael Henrickson (Children’s Hospital, Madera, CA), Lisa Imundo (Columbia University, New York, NY), Ildy M. Katona (Uniformed Services University, Bethesda, MD), Carol B. Lindsley (University of Kansas, Kansas City), Chester P. Oddis (University of Pittsburgh, Pittsburgh, PA), Judyann C. Olson (Medical College of Wisconsin, Milwaukee), David Sherry (Children’s Hospital of Philadelphia, Philadelphia, PA), Scott A. Vogelgesang (Walter Reed Army Medical Center, Washington, DC), Carol A. Wallace (Children’s Medical Center, Seattle, WA), Patience H. White (George Washington University, Washington, DC), and Lawrence S. Zemel (Connecticut Children’s Hospital, Hartford).
Footnotes
Supplemental materials include extended methods, five figures and 15 tables.
Reference List
- 1.Rider LG, Miller FW. Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA. 2011;305:183–90. doi: 10.1001/jama.2010.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Love LA, Leff RL, Fraser DD, Targoff IN, Dalakas M, Plotz PH, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991;70:360–74. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Chinoy H, Platt H, Lamb JA, Betteridge Z, Gunawardena H, Fertig N, et al. The protein tyrosine phosphatase N22 gene is associated with juvenile and adult idiopathic inflammatory myopathy independent of the HLA 8.1 haplotype in British Caucasian patients. Arthritis Rheum. 2008;58:3247–54. doi: 10.1002/art.23900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller FW, Cooper RG, Vencovsky J, Rider LG, Danko K, Wedderburn LR, et al. Genome-wide association study of dermatomyositis reveals genetic overlap with other autoimmune disorders. Arthritis Rheum. 2013;65:3239–47. doi: 10.1002/art.38137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jani M, Massey J, Wedderburn LR, Vencovsky J, Danko K, Lundberg IE, et al. Genotyping of immune-related genetic variants identifies TYK2 as a novel associated locus for idiopathic inflammatory myopathies. Ann Rheum Dis. 2014;73:1750–2. doi: 10.1136/annrheumdis-2014-205440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trynka G, Hunt KA, Bockett NA, Romanos J, Mistry V, Szperl A, et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat Genet. 2011;43:1193–201. doi: 10.1038/ng.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 8.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 9.Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, Mendell JR, et al. Inclusion body myositis and myopathies. Ann Neurol. 1995;38:705–13. doi: 10.1002/ana.410380504. [DOI] [PubMed] [Google Scholar]
- 10.Rose MR. 188th ENMC International Workshop: Inclusion Body Myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Disord. 2013;23:1044–55. doi: 10.1016/j.nmd.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Hilton-Jones D, Miller A, Parton M, Holton J, Sewry C, Hanna MG. Inclusion body myositis: MRC Centre for Neuromuscular Diseases, IBM workshop, London, 13 June 2008. Neuromuscul Disord. 2010;20:142–7. doi: 10.1016/j.nmd.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Connors GR, Christopher-Stine L, Oddis CV, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest. 2010;138:1464–74. doi: 10.1378/chest.10-0180. [DOI] [PubMed] [Google Scholar]
- 13.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7(Unit7) doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–81. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 15.Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. 2005:1034–50. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper JD, Simmonds MJ, Walker NM, Burren O, Brand OJ, Guo H, et al. Seven newly identified loci for autoimmune thyroid disease. 2012:5202–8. doi: 10.1093/hmg/dds357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinks A, Cobb J, Marion MC, Prahalad S, Sudman M, Bowes J, et al. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat Genet. 2013;45:664–9. doi: 10.1038/ng.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41:1228–33. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayes MD, Bossini-Castillo L, Gorlova O, Martin JE, Zhou X, Chen WV, et al. Immunochip analysis identifies multiple susceptibility loci for systemic sclerosis. Am J Hum Genet. 2014;94:47–61. doi: 10.1016/j.ajhg.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–7. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Y, Birlea SA, Fain PR, Ferrara TM, Ben S, Riccardi SL, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676–80. doi: 10.1038/ng.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44:1341–8. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JZ, Almarri MA, Gaffney DJ, Mells GF, Jostins L, Cordell HJ, et al. Dense fine-mapping study identifies new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2012;44:1137–41. doi: 10.1038/ng.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45:670–5. doi: 10.1038/ng.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren’s syndrome. Nat Genet. 2013;45:1284–92. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45:1353–60. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44:1336–40. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onengut-Gumuscu S, Chen WM, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47:381–6. doi: 10.1038/ng.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li MX, Yeung JM, Cherny SS, Sham PC. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum Genet. 2012;131:747–56. doi: 10.1007/s00439-011-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeller T, Wild P, Szymczak S, Rotival M, Schillert A, Castagne R, et al. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PLoS One. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, Beazley C, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–24. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veyrieras JB, Kudaravalli S, Kim SY, Dermitzakis ET, Gilad Y, Stephens M, et al. High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genet. 2008;4:e1000214. doi: 10.1371/journal.pgen.1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montgomery SB, Sammeth M, Gutierrez-Arcelus M, Lach RP, Ingle C, Nisbett J, et al. Transcriptome genetics using second generation sequencing in a Caucasian population. Nature. 2010;464:773–7. doi: 10.1038/nature08903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimas AS, Deutsch S, Stranger BE, Montgomery SB, Borel C, ttar-Cohen H, et al. Common regulatory variation impacts gene expression in a cell type-dependent manner. Science. 2009;325:1246–50. doi: 10.1126/science.1174148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, et al. Annotation of functional variation in personal genomes using RegulomeDB. 2012:1790–7. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang TP, Beazley C, Montgomery SB, Dimas AS, Gutierrez-Arcelus M, Stranger BE, et al. Genevar: a database and Java application for the analysis and visualization of SNP-gene associations in eQTL studies. Bioinformatics. 2010;26:2474–6. doi: 10.1093/bioinformatics/btq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vang T, Congia M, Macis MD, Musumeci L, Orru V, Zavattari P, et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–9. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Zahir N, Jiang Q, Miliotis H, Heyraud S, Meng X, et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43:902–7. doi: 10.1038/ng.904. [DOI] [PubMed] [Google Scholar]
- 41.Sugiura T, Kawaguchi Y, Goto K, Hayashi Y, Tsuburaya R, Furuya T, et al. Positive association between STAT4 polymorphisms and polymyositis/dermatomyositis in a Japanese population. Ann Rheum Dis. 2012;71:1646–50. doi: 10.1136/annrheumdis-2011-200839. [DOI] [PubMed] [Google Scholar]
- 42.Armstrong DL, Zidovetzki R, arcon-Riquelme ME, Tsao BP, Criswell LA, Kimberly RP, et al. GWAS identifies novel SLE susceptibility genes and explains the association of the HLA region. Genes Immun. 2014;15:347–54. doi: 10.1038/gene.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis MJ, Vyse S, Shields AM, Boeltz S, Gordon PA, Spector TD, et al. UBE2L3 polymorphism amplifies NF-kappaB activation and promotes plasma cell development, linking linear ubiquitination to multiple autoimmune diseases. Am J Hum Genet. 2015;96:221–34. doi: 10.1016/j.ajhg.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugiura T, Kawaguchi Y, Goto K, Hayashi Y, Gono T, Furuya T, et al. Association between a C8orf13-BLK polymorphism and polymyositis/dermatomyositis in the Japanese population: an additive effect with STAT4 on disease susceptibility. PLoS One. 2014;9:e90019. doi: 10.1371/journal.pone.0090019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faraco J, Lin L, Kornum BR, Kenny EE, Trynka G, Einen M, et al. ImmunoChip study implicates antigen presentation to T cells in narcolepsy. PLoS Genet. 2013;9:e1003270. doi: 10.1371/journal.pgen.1003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia X, Han B, Onengut-Gumuscu S, Chen WM, Concannon PJ, Rich SS, et al. Imputing amino acid polymorphisms in human leukocyte antigens. PLoS One. 2013;8:e64683. doi: 10.1371/journal.pone.0064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menconi F, Osman R, Monti MC, Greenberg DA, Concepcion ES, Tomer Y. Shared molecular amino acid signature in the HLA-DR peptide binding pocket predisposes to both autoimmune diabetes and thyroiditis. Proc Natl Acad Sci U S A. 2010;107:16899–903. doi: 10.1073/pnas.1009511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.